Business Editors/Health/Medical Writers
BRIDGEWATER, N.J.--(BUSINESS WIRE)--Feb. 2, 2004
Enzon Pharmaceuticals, Inc. (NASDAQ:ENZN) announced today that the Company has entered into an agreement to co-promote Bio-Rad Laboratories, Inc. (AMEX:BIO and BIOb) Platelia(TM) Aspergillus EIA test kit, a non-invasive diagnostic test that can improve the overall recognition and diagnosis of Invasive Aspergillosis. The Platelia(TM) Aspergillus EIA test kit is complementary to Enzon's product ABELCET(R). ABELCET (Amphotericin B Lipid Complex Injection) is a potent, broad-spectrum, fungicidal agent that is currently the most widely used treatment for invasive fungal infections among the lipid formulations of amphotericin B.
Enzon's ABELCET sales force will co-promote Bio-Rad's Aspergillus EIA test kit throughout hospitals in the United States and Canada, providing Bio-Rad with greater exposure to physicians that are treating patients with the highest risk of developing this serious fungal infection. Remuneration to Enzon was not disclosed.
"The combination of Bio-Rad's powerful diagnostic tool with ABELCET's proven track record as an effective antifungal agent will enable our sales team to provide physicians with a premier package aimed at total patient care," said Clarke Atwell, Enzon Vice President of Marketing and Sales.
"We are pleased to be able to partner with Enzon to offer laboratories and physicians a dramatic improvement to traditional methods of diagnosing Invasive Aspergillosis," said John Goetz, Bio-Rad Vice President of Clinical Diagnostics.
About Platelia Aspergillus EIA Test Kit
Bio-Rad's Platelia(TM) Aspergillus EIA test kit is a non-invasive diagnostic method that helps identify Aspergillus species rapidly and specifically when used in conjunction with other test procedures. The test kit can diagnose Aspergillus infection in as little as 3 hours, compared to 2 to 3 days for traditional methods. This provides the ability to detect Aspergillus species early when treatment can be more effective, from 6-13 days before clinical symptoms occur. Bio-Rad received US Food and Drug Administration (FDA) clearance to market the breakthrough Aspergillus test in May 2003. It is the first test available in the United States for invasive aspergillosis, a potentially life-threatening fungal infection affecting immunocompromised patients such as those suffering from cancer, HIV, recipients of lung and bone marrow transplants, and others. The infection has been difficult to diagnose, as traditional laboratory methods lack the sensitivity and specificity needed to produce results that are conclusive, and traditional testing can be physically intrusive and time-consuming. The test has been used throughout Europe and elsewhere in the world for the past 6 years.
About Invasive Aspergillosis
Invasive Aspergillosis is an invasive fungal infection that often affects patients with compromised immune systems, such as those suffering from cancer, HIV, and recipients of lung or bone marrow transplants. The Invasive Aspergillosis mortality rate approaches 100%, largely due to difficulties in diagnosing the disease reliably and in a timely manner, before fungal proliferation becomes overwhelming. Early diagnosis is critical for effective treatment and can mean the difference between life and death.
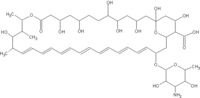
About ABELCET (amphotericin B lipid complex injection)
ABELCET was approved for use by the U.S. Food and Drug Administration (FDA) in November 1995. It is a lipid-based formulation of amphotericin B. Abelcet is the market leader among the 3 commercially available lipid-based formulations of amphotericin B. ABELCET offers clinicians the efficacy of amphotericin B combined with a greatly improved safety profile. ABELCET is indicated for the treatment of invasive fungal infections in patients who are refractory to or intolerant of conventional amphotericin B therapy. The adverse events most commonly reported with ABELCET are transient chills and/or fever during infusion of the drug. ABELCET is contraindicated in patients who have shown hypersensitivity to amphotericin B or any other component in the formulation. Please see full prescribing information before using ABELCET or any product mentioned in this press release.
About Bio-Rad Laboratories
Bio-Rad Laboratories, Inc. (www.bio-rad.com) is a multinational manufacturer and distributor of life science research products and clinical diagnostics. It is based in Hercules, CA, and serves more than 70,000 research and industry customers worldwide through a network of more than 30 wholly owned subsidiary offices.
About Enzon Pharmaceuticals
Enzon Pharmaceuticals is a biopharmaceutical company dedicated to the discovery, development and commercialization of therapeutics to treat life-threatening diseases. The company has developed or acquired a number of marketed products, including PEG-INTRON, marketed by Schering-Plough, and ABELCET, which is marketed in North America by Enzon. Enzon's science-focused strategy includes an extensive drug development program that leverages the Company's macromolecular engineering platform, including PEG modification and single-chain antibody (SCA(R)) technologies. Internal research and development efforts are complemented by strategic transactions that provide access to additional products, projects, and technologies. Enzon has several drug candidates in various stages of development, independently and with partners.
There are forward-looking statements contained herein that are not based on historical fact, including without limitation statements containing the words "believes," "may," "plans," "will," "estimate," "continue," "anticipates," "intends," "expects," and similar expressions. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause actual results, events or developments to be materially different from the future results, events or developments discussed above. Such factors include those described in the Company's Form 10-K and Forms 10-Q on file with the SEC, including without limitation, Enzon's ability to continue to increase ABELCET's share of the antifungal market and to sustain such increased market share and to successfully market its proprietary products; market acceptance of and continuing demand for Enzon's products; the uncertainty of the timing and results of clinical trials and the impact of competitive products and pricing. All information in this press release is as of February 2, 2004, and the Company undertakes no duty to update this information.
This release is also available at http://www.enzon.com
COPYRIGHT 2004 Business Wire
COPYRIGHT 2004 Gale Group