Medications for treating alcohol dependence primarily have been adjunctive interventions, and only three medications--disulfiram, naltrexone, and acamprosate--are approved for this indication by the U.S. Food and Drug Administration. Disulfiram, an aversive agent that has been used for more than 40 years, has significant adverse effects and compliance difficulties with no clear evidence that it increases abstinence rates, decreases relapse rates, or reduces cravings. In contrast, naltrexone, an anticraving agent, reduces relapse rates and cravings and increases abstinence rates. Acamprosate also reduces relapse rates and increases abstinence rates. Serotonergic and anticonvulsant agents promise to play more of a role in the treatment of alcohol dependence. Although not approved by the U.S. Food and Drug Administration for this indication, the anticonvulsant topiramate and several serotonergic agents (e.g., fluoxetine, ondansetron) have been shown in recent studies to increase abstinence rates and decrease drinking.
**********
Almost one third of Americans consume enough alcohol to be considered at risk for alcohol dependence, and alcohol abuse and dependence are associated with more than 100,000 deaths from alcohol-related diseases and injuries each year. The economic cost of alcohol abuse and dependence was estimated at more than $184 billion for 1998. (1) Use of screening tools and brief primary care interventions for alcohol problems significantly reduces drinking levels in "problem drinkers" who are not yet alcohol dependent. (2) Counseling and 12-step structured treatment programs have been the mainstays of alcohol dependence treatment, whereas pharmacologic treatments traditionally have played an adjunctive role.
To date, three medications--disulfiram (Antabuse), naltrexone (Trexan), and acamprosate (Campral)--have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of alcohol dependence, and only about 20 percent of eligible patients receive them. In the past decade, however, there has been a growing body of evidence supporting a more central role for medications in the treatment of alcohol dependence. These medications, the evidence supporting them, and recommended dosages are discussed in the following. Table 1 (3,4) provides a summary of the medications with prescribing information, adverse effects, contraindications, and costs.
Naltrexone
Naltrexone is an opioid-receptor antagonist approved for use in the treatment of alcohol dependence in conjunction with psychosocial interventions. It is believed that naltrexone works through its blockage of [micro]-opioid receptors, which reduces the reinforcing effects of alcohol leading to decreased feelings of intoxication and fewer cravings.
In a systematic review (5) of 11 double-blind, placebo-controlled trials, researchers found that naltrexone reduces short-term relapse rates in patients with alcohol dependence when combined with psychosocial treatments. Short-term outcomes in favor of naltrexone included fewer patients relapsing to alcohol dependence (38 versus 60 percent with placebo), fewer patients returning to drinking (61 versus 69 percent), reduced cravings for alcohol, and fewer drinking days. (5) The data showed one relapse was prevented for every five patients treated with naltrexone (i.e., number needed to treat [NNT] = 5).
More recent randomized controlled trials (RCTs) looking at longer-term outcomes report mixed results. In a systematic review (6) of three studies assessing medium-term outcomes (six to 12 months), researchers found no difference between naltrexone and placebo groups. In addition, a large trial (7) comparing outcomes of three therapy groups--12 months of naltrexone therapy, three months of naltrexone followed by nine months of placebo, and 12 months of placebo--found no significant differences among the groups in the number of days to relapse, number of drinking days, or number of drinks per drinking day. Although there is good evidence supporting short-term benefit with naltrexone, the evidence for longer-term use is less compelling.
The recommended dosage of naltrexone is 50 mg per day in a single dose. Long-term opioid therapy for chronic pain or heroin dependence is a contraindication for naltrexone because the drug could precipitate severe withdrawal syndrome. Naltrexone has been shown to have dose-related hepatotoxicity, although generally this occurs at doses higher than those recommended for treatment of alcohol dependence. The drug also is contraindicated in patients with hepatitis or liver failure, and all patients should have hepatic transaminase levels checked monthly for the first three months and every three months thereafter. (8)
Naltrexone generally is well tolerated; nausea is the most common adverse effect (reported by 10 percent of patients), followed by headache, anxiety, and sedation. (9)
Naltrexone is FDA pregnancy category C. Good compliance is considered essential for successful treatment.
Disulfiram
Disulfiram inhibits acetaldehyde dehydrogenase. Although it has been used to treat alcohol dependence for more than 40 years, the evidence for its effectiveness is weak. An evidence report from the Agency for Healthcare Research and Quality (6) concluded that studies using the disulfiram implant display serious methodologic weaknesses (most substantively, regarding the question of bioavailability), and that the four placebo-controlled RCTs using oral disulfiram produced mixed results. Although in two trials oral disulfiram was shown to reduce frequency of drinking days, it did not improve relapse rates compared with placebo. Two studies noted patient compliance with oral disulfiram and showed it to be low, and a third study had a 46 percent dropout rate. These methodologic limitations and mixed results make it difficult to state clearly how many patients benefit from disulfiram.
Disulfiram usually is given in a dosage of 250 mg per day with a maximum dosage of 500 mg per day. Consuming alcohol after taking disulfiram results in symptoms such as palpitations, flushing, nausea, vomiting, and headache. More severe reactions could include myocardial infarction, congestive heart failure, respiratory depression, and death. Because of the potential for a severe alcohol-disulfiram interaction, disulfiram is contraindicated in patients who are receiving or have recently received metronidazole or ingested alcohol, have psychosis, or have cardiovascular disease, and is not recommended for patients with severe pulmonary disease, chronic renal failure, or diabetes, or those older than 60 years. It also is not recommended in patients with peripheral neuropathy, seizures, or cirrhosis with portal hypertension. Hepatotoxicity is a rare but potentially fatal adverse effect. (8) Some experts recommend that baseline liver function tests should be obtained, with repeat testing at two weeks, three months, six months, and then every six months thereafter. Because of these significant restrictions and problems with compliance, disulfiram is not recommended for treating alcohol dependence, particularly in the primary care setting. (6) Disulfiram is FDA pregnancy category C.
Acamprosate
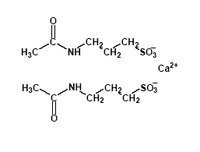
Acamprosate (calcium homotaurinate) is believed to block glutaminergic N-methyl-Daspartate receptors and activate [lambda]-aminobutyric acid type A receptors, and was recently approved by the FDA for the treatment of alcohol dependence. A systematic review (10) of 15 studies showed that acamprosate reduces short-term and long-term (more than six months) relapse rates in patients with alcohol dependence when combined with psychosocial treatments. Outcomes in favor of acamprosate included fewer patients returning to drinking (68 versus 80 percent, NNT = 8) and higher percentage of days of total abstinence (54 versus 38 percent, NNT = 7). (10)
Acamprosate is available in 333-mg enteric, coated tablets; dosing is by weight (Table 1 (3,4)). It is well tolerated with limited side effects, most commonly transient diarrhea (occurring in approximately 10 percent of patients). There are no interactions with concomitant use of alcohol, diazepam (Valium), disulfiram, or imipramine (Tofranil), so patients with alcohol dependence can continue to use acamprosate during a relapse. Patients with renal insufficiency or advanced cirrhosis should not take acamprosate, but it may be taken safely by patients with liver dysfunction. 11 Like naltrexone and disulfiram, acamprosate is FDA pregnancy category C (adverse effects on the fetus in animal studies but no human trials).
Serotonergic Agents
Research into the use of selective serotonin reuptake inhibitors (SSRIs) to treat patients with alcohol dependence has been under way for the past decade; however, most of this work has used small samples and inconsistent outcome measures. One clinical trial (12) of 101 patients showed that fluoxetine (Prozac) at a dosage of up to 60 mg per day had no significant effect on alcohol consumption in persons who were alcohol dependent without major depression. In a study (13) involving psychiatric patients with major depression and alcohol dependence, those treated with 20 to 40 mg per day of fluoxetine over 12 weeks had fewer drinks, fewer drinking days, and fewer heavy drinking days than those receiving placebo. Other SSRIs have shown similar results. (14) Studies on the effect of SSRIs in patients with more severe alcohol dependence (Type B according to the classification system by Babor and colleagues (15)) show no clear benefit and sometimes show trends toward worse outcomes with SSRIs, (16) and studies involving patients with less severe alcohol dependence (Babor Type A15) show no consistent benefit. (17)
The use of selective serotonin antagonists for early-onset alcohol dependence also has been investigated, with positive results. In one RCT, (3)(ondansetron (Zofran) was shown to significantly reduce self-reported drinking. Patients who received ondansetron 4 mcg per kg twice per day had fewer drinks per day. They also had a greater percentage of days of abstinence (70 versus 50 percent with placebo) and a greater total number of days abstinent per study week (6.7 versus 5.9 with placebo) in patients with early-onset alcoholism. All patients also received weekly group cognitive behavior therapy.
Serotonergic agents generally are well tolerated. (18) Nausea, headache, sedation, and sexual dysfunction are among the most commonly reported adverse effects. The most significant drug interactions for SSRIs are with monoamine oxidase inhibitors, warfarin (Coumadin), some antipsychotics, tetracyclic antidepressants, some benzodiazepines, St. John's wort, and phenytoin (Dilantin). (18)
Anticonvulsants
Recent research suggests a role for anticonvulsants in the treatment of alcohol dependence beyond their use in withdrawal syndromes. (4) Topiramate (Topamax) inhibits mesocorticolimbic dopamine release, which is believed to be associated with craving for alcohol. It is the best-studied drug in this class, although gabapentin (Neurontin) (19) and valproate (Depacon) (20) have shown some promise in case studies and small trials.
In a 12-week double-blind RCT (4) of actively drinking patients with alcohol dependence, topiramate was more effective than placebo in initiating abstinence (26 percent more abstinent days with topiramate) and in reducing self-reported drinks per day, drinks per drinking day, and heavy drinking days. Compared with placebo, it also significantly reduced craving as measured on an obsessive-compulsive-drinking scale. These findings occurred in patients with early-onset and late-onset alcoholism.
The study (4) used an escalating dose of 25 to 300 mg of topiramate per day. Hypersensitivity to the drug is the only known contraindication. Adverse effects include dizziness and somnolence (which are not dose related), ataxia, impaired concentration, confusion, fatigue, paresthesias, speech difficulties, diplopia, and nausea. Interactions exist between topiramate and other anticonvulsants, including phenytoin, valproic acid (Depakene), and carbamazepine (Tegretol).
Nalmefene
Nalmefene (Revex), another opioid antagonist, is similar to naltrexone but without FDA approval for treatment of alcohol dependence. Nalmefene at dosages of 20 or 80 mg orally per day has been shown in one RCT (21) to significantly reduce relapse to heavy drinking in outpatients with alcohol dependence. Patients receiving nalmefene had a 37 percent relapse rate compared with 59 percent in the placebo group (NNT = 5). However, treatment groups did not differ significantly in the percentage of days abstinent, in the mean number of drinks consumed in a drinking day, or in self-reported craving ratings. This 12-week study (21) also provided weekly cognitive behavior therapy to all groups. Outside of research, nalmefene is available only in an injectable form.
Final Comments
The best choices for prevention of relapse are acamprosate and naltrexone with concurrent counseling through professional or self-help programs. Family physicians also may consider the use of an SSRI in the presence of a comorbid mood disorder. Evidence is lacking for combination pharmacotherapy, but research is under way. Topiramate and ondansetron show promise as treatments to increase abstinence. Because of its lack of effectiveness and problems with adverse effects and compliance, disulfiram is not recommended in the primary care setting.
Author disclosure: Nothing to disclose.
NOTE: Studies for this article were identified through a systematic search of the Cochrane and ETOH databases and PubMed for articles published from 1996 to 2003 using the subject headings alcoholism and drug therapy and the drug names. The review also included bibliographies of the identified articles.
This article is one in a series coordinated by Allen F. Shaughnessy, Pharm.D., Tufts University Family Medicine Residency, Malden, Me.
REFERENCES
(1.) Harwood HJ. Updating estimates of the economic costs of alcohol abuse in the United States: estimates, update methods, and data. Bethesda, Md.: U.S. Department of Health and Human Services; National Institute on Alcohol Abuse and Alcoholism, 2000.
(2.) Beich A, Thorsen T, Rollnick S. Screening in brief intervention trials targeting excessive drinkers in general practice: systematic review and meta-analysis. BMJ 2003;327:536-42.
(3.) Johnson BA, Roache JD, Javors MA, DiClemente CC, Cloninger CR, Prihoda TJ, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA 2000;284:963-71.
(4.) Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet 2003;361:1677-85.
(5.) Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev;(1): CD001867.
(6.) West SL, Garbutt JC, Carey TS, Lux LJ, Jackman AM, Tolleson-Rinehart S, et al. Pharmacotherapy for alcohol dependence. Rockville, Md.: U.S. Department of Health and Human Services; Public Health Service; Agency for Health Care Policy and Research, 1999.
(7.) Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med 2001;345:1734-9.
(8.) Doering PL. Substance-related disorders: alcohol, nicotine, and caffeine. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, eds. Pharmacotherapy: a pathophysiologic approach. 4th ed. Stamford, Conn.: Appleton & Lange, 1999.
(9.) Croop RS, Faulkner EB, Labriola DF. The safety profile of naltrexone in the treatment of alcoholism: results from a multicenter usage study. Arch Gen Psychiatry 1997;54:1130-5.
(10.) Mason BJ. Treatment of alcohol-dependent outpatients with acamprosate: a clinical review. J Clin Psychiatry 2001;62(suppl 20):42-8.
(11.) Graham R, Wodak AD, Whelan G. New pharmacotherapies for alcohol dependence. MJA 2002;177:103-7.
(12.) Kranzler HR, Burleson JA, Korner P, Del Boca FK, Bohn MJ, Brown J, et al. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry 1995;152:391-7.
(13.) Cornelius JR, Salloum IM, Ehler JG, Jarrett PJ, Cornelius MD, Perel JM, et al. Fluoxetine in depressed alcoholics: a double-blind, placebo-controlled trial. Arch Gen Psychiatry 1997;54:700-5.
(14.) Naranjo CA, Knoke DM. The role of selective serotonin reuptake inhibitors in reducing alcohol consumption. J Clin Psychiatry 2001;62(suppl 20):18-25.
(15.) Babor TF, Dolinsky ZS, Meyer RE, Hesselbrock M, Hofmann M, Tennen H. Types of alcoholics: concurrent and predictive validity of some common classification schemes. Br J Addict 1992;87:1415-31.
(16.) Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res 1996;20:1534-41.
(17.) Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and subtype. Alcohol Clin Exp Res 2000;24:1041-9.
(18.) Bezchlibnyk-Butler KZ, Jeffries JJ, Martin BA. Clinical handbook of psychotropic drugs. 10th ed. Seattle: Hogrefe & Huber, 2000.
(19.) Chatterjee CR, Ringold AL. A case report of reduction in alcohol craving and protection against alcohol withdrawal by gabapentin. J Clin Psychiatry 1999;60:617.
(20.) Brady KT, Myrick H, Henderson S, Coffey SF. The use of divalproex in alcohol relapse prevention: a pilot study. Drug Alcohol Depend 2002;67:323-30.
(21.) Mason BJ, Salvato FR, Williams LD, Ritvo EC, Cutler RB. A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Arch Gen Psychiatry 1999;56:719-24.
STEVEN H. WILLIAMS, PH.D., is former director of behavioral medicine at the Harrisburg (Pa.) Family Practice Residency Program and currently is a clinical psychologist with the Psychology Service at the Veterans Affairs Medical Center in Lebanon, Pa. Dr. Williams received a doctoral degree in counseling psychology from the University of Florida, Gainesville, and a postdoctoral certification in psychopharmacology from Fairleigh Dickinson University, Teaneck, N.J.
Address correspondence to Steven H. Williams, Ph.D., VA Medical Center, 1700 S. Lincoln Ave., Lebanon, PA 17042. Reprints are not available from the author.
COPYRIGHT 2005 American Academy of Family Physicians
COPYRIGHT 2005 Gale Group