ABSTRACT Poly(ethylene glycol)^sub 2000^C^sub 20^ceramide (PEG-Cer) containing monolayers at an air/water interface were characterized by measuring their surface pressure versus area/molecule (pi-A) and surface potential versus area/molecule (DeltaV-A) isotherms. The behavior of pi-A as well as DeltaV versus lipid density (DeltaV-n) and DeltaV-pi isotherms for PEG-Cer are in keeping with two transitions of the lipopolymer, starting at pi= 9 and 21 mN/m. We also investigated the effects of PEG-Cer on the binding of adriamycin, cytochrome c and bovine serum albumin to monolayers containing varying mole fractions X of PEG-Cer. PEG-Cer impedes the penetration of these ligands into lipid monolayers with similar effects at both X = 0.04 and 0.08. This effect of PEG-Cer depends on the conformation of the lipopolymer and the interactions between the lipid surface and the surface-interacting molecule as well as the size of the latter.
INTRODUCTION
Adsorbed or grafted hydrophilic polymers such as polyethylene glycol (PEG) immobilized at the interface between biofluids and biomaterials have gained considerable attention. This is because of their unique biological inertness, which is considered to result from hydrophilicity and chain mobility as well as lack of ionic charges (Desai and Hubbell, 1991). This inertness allows construction of biocompatible surfaces (for reviews see Torchilin et al., 1995; Woodle, 1995; Sadzuka, 2000). In addition to efforts aiming at practical applications, these polymers have been subjected to both theoretical (Alexander, 1977; de Gennes, 1980; Jeon et al., 1991; Szleifer, 1997a; Halperin, 1999) and experimental (Du et al., 1997; Wong et al., 1997; Majewski et al., 1998; Baekmark et al., 1995, 1999; Wiesenthal et al., 1999; Naumann et al., 1999) studies. Polymer-modified lipids serve as good models for grafted polymers of low molecular weight, where the grafting density of the polymer chains can be varied and quantitatively controlled by simply varying the ratio of unmodified to polymer-modified lipid within a mixed monolayer or a bilayer (Kuhl et al., 1994; Kenworthy et al., 1995; Majewski et al., 1997). Inclusion of phospholipids with grafted PEG chains into phospholipid liposomes (forming so-called stealth liposomes) prolongs their half-time in circulation and increases their efficiency in drug delivery (for reviews, see Torchilin et al., 1995; Woodle, 1995; Sadzuka, 2000). This effect has been attributed to the repulsive hydrophilic barrier around the liposome provided by the covalently attached PEG, which prevents liposomes from cell adhesion and from being opsonized by proteins (Senior et al., 1991; Du et al., 1997).
The above effect of PEG-conjugated lipids has been recognized to depend on the molecular weight of the PEG moiety as well as on the density of grafted PEG on the membranes (Kenworthy et al., 1995). Also the structure of the lipid anchor is important (Webb et al., 1998; AdlakhaHutcheon et al., 1999). Leakage of the anticancer drug vincristine from liposomes containing PEG-ceramide (PEGCer) is less than from liposomes containing 1,2-distearoylsn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)] (PEG-DSPE) (Webb et al., 1998). The longer ceramide acyl chains seem to provide more efficient anchoring to the liposomes. PEG-conjugated ceramides have been demonstrated to promote bilayer formation in mixtures with non-bilayer-forming lipids (Holland et al., 1996a) and to regulate fusion of liposomes as well as liposomes and cells (Holland et al., 1996b).
A close simulation of PEG-liposome surfaces is a lipid monolayer or bilayer on a solid support with the grafted PEG moieties protruding from the surface and the hydrophobic tails of these molecules remaining inserted into the surface monolayer (Majewski et al., 1998; Kuhl et al., 1998; Baekmark et al., 1995, 1999). Lipid monolayers at the air/water interface have well-defined composition as well as lateral packing density and allow us to study various processes such as drug-ligand and protein-lipid interactions in the membrane/water interface under precisely controlled conditions (for review see Brockman, 1999). Recently, polymer-grafted lipids (lipopolymers) and their mixtures with different lipids were subjected to Langmuir-balance studies (Baekmark et al., 1995, 1999; Majewski et al., 1998) and were found to form stable films that exhibit a complex phase behavior (Baekmark et al., 1997; Wiesenthal et al., 1999; Naumann et al., 1999).
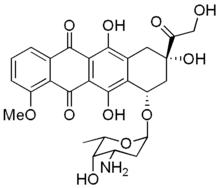
Adsorption of drugs and proteins into membrane surfaces and their behavior at interfaces as well as interactions with lipids are of interest in relation to cell membrane organization and functions (for reviews see Kinnunen, 1991; Kinnunen et al., 1994). In this study we compared the binding of three soluble molecules, adriamycin, cytochrome c, and bovine serum albumin (BSA) to PEG-Cer-containing monolayers. Adriamycin is a commonly used anticancer drug that bears a positive charge and interacts strongly with membranes containing acidic phospholipids (Goormaghtigh et al., 1980; De Wolf et al., 1991; Mustonen and Kinnunen, 1991). Adriamycin decreases acyl chain order in an acidic phospholipid membrane, thus implying disruption of the local membrane structure and altered physical state of membrane lipids (De Wolf et al., 1991). These membrane interactions could result in changes in lipid organization, and may also play a role in the antitumor activity of this drug (De Wolf et al., 1991). Membrane penetration of adriamycin is strongly dependent on lipid packing (Mustonen and Kinnunen, 1993). Drug-lipid interactions also contribute to efficiency of encapsulation of adriamycin into vesicles (Hernandez et al., 1991).
Cytochrome c (cyt c) is a well-characterized peripheral protein of the inner mitochondrial membrane that associates only weakly with zwitterionic phosphatidylcholine membranes (Mustonen et al., 1993). In keeping with its net positive charge and the presence of cationic clusters on its surface cyt c binds with a high affinity to acidic phospholipids (for review see Kinnunen et al., 1994). Adriamycin has been shown to reverse the binding of cyt c to cardiolipin at equimolar drug-lipid concentrations (Goormaghtigh et al., 1982). It has been suggested that the association of adriamycin and cyt c with acidic lipids involves similar mechanisms, with both hydrophobic as well as electrostatic interactions being involved (Mustonen et al., 1993). Yet, hydrophobicity appears to contribute less to the membrane association of adriamycin. Intriguingly, recent results show that cyt c is also centrally involved in apoptosis (Kluck et al., 1997; Yang et al., 1997), its release from mitochondria representing the rate-limiting step in the commitment of a cell to programmed cell death (Liu et al., 1996; Kluck et al., 1997; Yang et al., 1997). The other protein investigated in the present study is BSA. It is considerably larger than cyt c, with a molecular weight of -66,000. BSA is the main component of plasma, constituting 50-60% of the total protein in blood. It promotes the aggregation and fusion of liposomes (Schenkman et al., 1981).
The technical assistance of Kaija Niva is appreciated.
This study was supported by the Finnish Academy and Technology Development Fund (TEKES).
REFERENCES
Adlakha-Hutcheon, G., M. B. Bally, C. R. Shew, and T. D. Madden. 1999. Controlled destabilization of a liposomal drug delivery system enhances mitoxantrone antitumor activity. Nat. Biotechnol 17:775-779.
Alexander, S. 1977. Adsorption of chain molecules with a polar head, a scaling description. J. Phys. (France). 38:983-987.
Baekmark, T. R., G. Elender, D. D. Lasic, and E. Sackmann. 1995. Conformational transitions of mixed monolayers of phospholipids and poly(ethylene oxide) lipopolymers and interaction forces with solid surfaces. Langmuir. 11:3975-3987.
Baekmark, T. R., T. Wiesenthal, P. Kuhn, A. Albersorfer, 0. Nuyken, and R. Merkel. 1999. A systematic infrared reflection-adsorption spectroscopy and film balance study of the phase behavior of lipopolymer monolayers at the air-water interface. Langmuir. 15:3616-3626.
Baekmark, T. R., T. Wiesenthal, P. Kuhn, T. M. Bayerl, 0. Nuyken, and R. Merkel. 1997. New insights into the phase behavior of lipopolymer monolayers at the air/water interface: IRRAS study of a polyoxazoline lipopolymer. Langmuir. 13:5521-5523.
Bijsterbosch, H. D., V. 0. de Haan, A. W. de Graaf, M. Mellema, F. A. M. Leermakers, M. A. Cohen Stuart, and A. A. van Well. 1995. Tethered adsorbing chains: neutron reflectivity and surface pressure of spread diblock copolymer monolayers. Langmuir. 11:4467-4473.
Blume, A. 1979. A comparative study of the phase transitions of phospholipid bilayers and monolayers. Biochim. Biophys. Acta. 557:32-44. Brockman, H. 1994. Dipole potential of lipid membranes. Chem. Phys. Lipids. 73:57-79.
Brockman, H. 1999. Lipid monolayers: why use half of a membrane to characterize protein-membrane interactions? Curr. Opin. Struct. BioL 9:438-443.
Carignano, M. A., and I. Szleifer. 1995. On the structure and pressure of tethered polymer layers in good solvent. Macromolecules. 28: 3197-3204.
de Gennes, P. G. 1980. Conformations of polymers attached to an interface. Macromolecules. 13:1069-1075.
Desai, N. P., and J. A. Hubbell. 1991. Solution technique to incorporate polyethylene oxide and other water-soluble polymers into surfaces of polymeric biomaterials. Biomaterials. 12:144-153.
De Wolf, F. A., M. Maliepaard, F. van Dorsten, I. Berghuis, K. Nicolay, and B. de Kruijff. 1991. Comparable interaction of doxorubicin with various acidic phospholipids results in changes of lipid order and dynamics. Biochim. Biophys. Acta. 1096:67-80.
Du, H., P. Chandaroy, and S. W. Hui. 1997. Grafted poly(ethylene glycol) on lipid surfaces inhibit protein adsorption and cell adhesion. Biochim. Biophys. Acta. 1326:236-248.
Faure, M. C., P. Bassereau, M. A. Carignano, I. Szleifer, Y. Gallot, and D. Andelman. 1998. Monolayers of diblock copolymer at the air/water interface: the attractive monomer-surface case. Eur. Phys. J. B. 3:365-375.
Fery-Forgues, S., J. P. Fayet, and A. Lopez. 1993. Drastic changes in the fluorescence properties of NBD probes with the polarity of the medium: involvement of TICT state? Photochem. Photobiol. A Chem. 70: 229-243.
Goormaghtigh, E., P. Chatelain, J. Caspers, and J. M. Ryusschaert. 1980. Evidence of a specific complex between adriamycin and negativelycharged phospholipids. Biochim. Biophys. Acta. 597:1-14.
Goormaghtigh, E., M. Vandenbranden, and J. M. Ruysschaert. 1982. Adriamycin inhibits the formation of non-bilayer lipid structures in cardiolipincontaining model membranes. Biochim. Biophys. Acta. 685:137-143.
Halperin, A. 1992. The phase behavior of tethered chains: an overview. Macromolecular Rep. A29:107-116
Halperin, A. 1999. Polymer brushes that resist adsorption of model proteins: design parameters. Langmuir. 15:2525-2533
Halperin, A., M. Tirrell, and T. P. Lodge. 1992. Tethered chains in polymer microstructures. Adv. Polymer Sci. 100:31-71.
Haydon, D. A., and J. L. Taylor. 1963. The stability and properties of bimolecular lipid leaflets in aqueous solution. J. Theor. Biol. 4:281-296. Hernandez, J., A. Marti, and J. Estelrich. 1991. Interaction of doxorubicin with lipid systems. Bioconjugate Chem. 2:398-402.
Holland, J. W., P. R. Cullis, and T. D. Madden. 1996a. Poly(ethylene glycol)-lipid conjugates promote bilayer formation in mixtures of nonbilayer-forming lipids. Biochemistry. 35:2610-2617.
Holland, J. W., C. Hui, P. R. Cullis, and T. D. Madden. 1996b. Poly(ethylene glycol)-lipid conjugates regulate the calcium-induced fusion of liposomes composed of phosphatidylethanolamine and phosphatidylserine. Biochemistry. 35:2618-2624.
Holopainen, J. M., H. L. Brockman, R. E. Brown, and P. K. J. Kinnunen. 2001. Interfacial interactions of ceramide with dimyristoylphophatidylcholine: impact of the N-acyl chain. Biophys. J. 80:765-775.
Jeon, S. I., J. H. Lee, J. D. Andrade, and P. G. de Gennes. 1991. Proteinsurface interactions in the presence of polyethylene oxide. I. Simplified theory. J. Colloid Interface Sci. 142:149-158.
Jeon, S. L, and J. D. Andrade. 1991. Proteins-surface interactions in the presence of polyethylene oxide. J. Colloid Interface Sci. 142:159-166. Kenworthy, K., K. Hristova, D. Needham, and T. J. McIntosh. 1995. Range
and magnitude of the steric pressure between bilayers containing phospholipids with covalently attached poly(ethylene glycol). Biophys. J. 68:1921-1936.
Kim, M. W., and B. H. Cao. 1993. Additional reduction of surface tension of aqueous polyethylene oxide (PEO) solution at high polymer concentration. Europhys. Lett. 24:229-234.
Kinnunen, P. K. J. 1991. On the principles of functional ordering in biological membranes. Chem. Phys. Lipids. 57:375-399.
Kinnunen, P. K. J., A. K6iv, J. Y. A. Lehtonen, M. Rytomaa, and P. Mustonen. 1994. Lipid dynamics and peripheral interactions of proteins with membrane surfaces. Chem. Phys. Lipids. 73:181-207.
Kluck, R. M., E. Bossy-Wetzel, D. R. Green, and D. D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 275:1132-1136.
Konttila, R., I. Salonen, J. A. Virtanen, and P. K. J. Kinnunen. 1988. Estimation of the equilibrium lateral pressure in liposomes of 1-palmitoyl-2-[ 10-(pyren- 1-yl)- 10-ketodecanoyl]-sn-glycero-3-phosphocholine and the effect of the phospholipid phase transition. Biochemistry. 27: 7443-7446.
Kuhl, T. L., D. E. Leckband, D. D. Lasic, and J. N. Israelachvili. 1994. Modulation of interaction forces between bilayers exposing shortchained ethylene oxide headgroup. Biophys. J. 66:1479-1488.
Kuhl, T. L., J. Majewski, J. Y. Wong, S. Steinberg, D. E. Leckband, J. N. Israelachvili, and G. S. Smith. 1998. A neutron reflectivity study of polymer-modified phospholipid monolayers at the solid-solution interface: polyethylene glycol-lipids on silane-modified substrates. Biophys. J. 75:2352-2362.
Leckband, D., S. Sheth, and A. Halperin. 1999. Grafted poly(ethylene oxide) brushes as nonfouling surface coatings. J. Biomater. Sci.-Polym. Ed. 10:1125-1147.
Liu, X., C. N. Kim, J. Yang, R. Jemerson, and X. Wang. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 86:147-157.
Majewski, J., T. L. Kuhl, M. C. Gerstenberg, J. N. Israelachvili, and G. S. Smith. 1997. Structure of phospholipid monolayers containing poly(ethylene glycol) lipids at the air-water interface. J. Phys. Chem. B. 101: 3122-3129.
Majewski, J., T. L. Kuhl, K. Kjaer, M. C. Gerstenberg, J. Als-Nielsen, J. N. Israelachvili, and G. S. Smith. 1998. X-ray synchrotron study of packing and protrusions of polymer-lipid monolayers at the air-water interface. J. Am. Chem. Soc. 120:1469-1473.
Menozzi, M., L. Valentini, E. Vannini, and F. Arcamone. 1984. Selfassociation of doxorubicin and related compounds in aqueous solution. J. Pharmacol. Sci. 73:766-770.
Milner, S. T. 1991. Polymer brushes. Science. 251:905-914.
Mustonen, P., and P. K. J. Kinnunen. 1991. Activation of phospholipase A2 by adriamycin in vitro. J. Biol. Chem. 266:6302-6307.
Mustonen, P., and P. K. J. Kinnunen. 1993. On the reversal by deoxyribonucleic acid of the binding of adriamycin to cardiolipin-containing liposomes. J. Biol. Chem. 268:1074-1080.
Mustonen, P., J. Lehtonen, A. Koiv, and P. K. J. Kinnunen. 1993. Effects of sphingosine on peripheral membrane interaction: comparison of adriamycin, cytochrome c, and phospholipase A2. Biochemistry. 32: 5373-5380.
Naumann, C. A., C. F. Brooks, G. G. Fuller, W. Knoll, and C. W. Frank. 1999. Viscoelastic properties of lipopolymers at the air-water interface: a combined interfacial stress rheometer and film balance study. Langmuir. 15:7752-7761.
Naumann, C. A., C. F. Brooks, G. Fuller, T. Lehmann, J. Ruhe, W. Knoll, P. Kuhn, 0. Nuyken, and C. W. Frank. 2001. Two dimensional physical networks of lipopolymers at the air/water interface: correlation of molecular structure and surface theological behavior. Langmuir. 17: 2801-2806.
Needham, D., T. J. McIntosh, and D. D. Lasic. 1992. Repulsive interactions and mechanical stability of polymer-grafted lipid membranes. Biochim. Biophys. Acta. 1108:40-48.
Quinn, P. J., and R. M. C. Dawson. 1970. An analysis of the interaction of protein with lipid monolayers at the air/water interface. Biochem. J. 116:671-680.
Sadzuka, Y. 2000. Effective prodrug liposome and conversion to active metabolite. Curr. Drug Metab. 1:31-48.
Schenkman, S., P. S. De Araujo, A. Sesso, F. H. Quina, and H. Chaimovich. 1981. A kinetic structural study of two-step aggregation and fusion of neutral phospholipid vesicles promoted by serum albumin at low pH. Chem. Phys. Lipids. 28:165-180.
Senior, J., C. Delgado, D. Fisher, C. Tilcock, and G. Gregoriadis. 1991. Influence of surface hydrophilicity of liposomes on their interaction with plasma protein and clearance from the circulation: studies with poly(ethylene glycol)-coated vesicles. Biochim. Biophys. Acta. 1062:77-82.
Szleifer, I. 1997a. Protein adsorption on surfaces with grafted polymers: a theoretical approach. Biophys. J. 72:595-612.
Szleifer, I. 1997b. Protein adsorption on tethered polymer layers: effect of polymer chains architecture and composition. Physica A. 244:370-388. Szleifer, I. 1997c. Polymers and proteins: interactions at interfaces. Curr. Opin. Solid State Mater. Sci. 2:337-344.
Torchilin, V. P., V. G. Omelyanenko, M. I. Papisov, A. A. Bogdanov Jr., V. S. Trubetskoy, J. N. Herron, and C. A. Gentry. 1994. Poly(ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochim. Biophys. Acta. 1195:11-20.
Torchilin, V. P., M. I. Papisov, A. A. Bogdanov, V. S. Trubetskoy, and V. G. Omelyanenko. 1995. Molecular mechanism of liposome and immunoliposome steric protection with poly(ethylene glycol): theoretical and experimental proofs of the role of polymer chain flexibility. Stealth Liposomes. 51-62.
Webb, M. S., D. S. Saxon, F. M. P. Wong, H. J. Lim, Z. Wang, M. B. Bally, S. L. Choi, P. R. Cullis, and L. D. Mayer. 1998. Comparison of different hydrophobic anchors conjugated to poly(ethylene glycol): ef
fects on the pharmacokinetics of liposomal vincristine. Biochim. Biophys. Acta. 1372:272-282.
Weis, R. M. 1991. Fluorescence microscopy of phospholipid monolayer phase transitions. Chem. Phys. Lipids. 57:227-239.
Wiesenthal, T., T. R. Baekmark, and R. Merkel. 1999. Direct evidence for a lipid alkyl chain ordering transition in poly(ethylene oxide) lipopolymer monolayers at the air/water interface obtained from infrared reflection absorption spectroscopy. Langmuir. 15:6837-6844.
Wong, J. Y., T. L. Kuhl, J. N. Israelachvili, N. Mullah, and S. Zalipsky. 1997. Direct measurement of a tethered ligand-receptor interaction potential. Science. 275:820-822.
Woodle, M. C. 1995. Sterically stabilized liposome therapeutics. Adv. Drug Delivery Rev. 16:249-265
Yang, J., X. Liu, K. Bhalla, C. N. Kim, A. M. Ibrado, J. Cai, T. L. Peng, D. P. Jones, and X. Wang. 1997. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 275:1129-1132.
Hongxia Zhao, Patricia M. Dubielecka, Tim Soderlund, and Paavo K. J. Kinnunen
Helsinki Biophysics and Biomembrane Group, Institute of Biomedicine, University of Helsinki, FIN-00014 Helsinki, Finland
Submitted September 4, 2001, and accepted for publication April 17, 2002. Address reprint requests to Dr. Paavo K.J. Kinnunen, Helsinki Biophysics and Biomembrane Group, Institute of Biomedicine, P.O. Box 63 (Haartmaninkatu 8), FIN-00014 University of Helsinki, Finland. Tel.: 358-919125400; Fax: 358-9-19125444; E-mail: paavo.kinnunen@helsinki.fi.
Copyright Biophysical Society Aug 2002
Provided by ProQuest Information and Learning Company. All rights Reserved