Cancer of the mesothelium--a membrane that covers and protects most of the body's internal organs--is usually associated with a history of asbestos exposure and affects the lining of the chest. About 2,000 new cases are diagnosed each year. By the time symptoms appear, the disease is often advanced, with people living nine to 13 months on average after diagnosis.
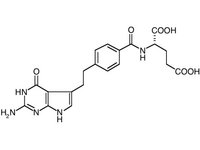
In February 2004, the FDA approved Alimta (pemetrexed), the first drug for a rare type of cancer called malignant pleural mesothelioma.
Alimta works by blocking enzymes thought to play a role in the rapid growth of lung tumors. The drug received a priority review and was approved for use with another cancer treatment called cisplatin. In a clinical trial, people receiving Alimta and cisplatin lived three months longer than those given cisplatin alone (12 months versus nine months). Alimta must be given with vitamin B-12 and folio acid to lower the chance and the severity of side effects.
The most common side effects are low white blood count, nausea, vomiting, fatigue, rash, and diarrhea. Alimta will be distributed by Eli Lilly and Company of Indianapolis.
COPYRIGHT 2004 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group