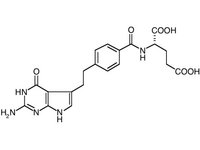
Abbreviation: COX-2 = cyelooxygenase-2
It is both an honor and a challenge to summarize this year's Aspen Lung Conference, organized by York Miller and Bob Keith, with the capable assistance of Jeanne Cleary. They haw, managed to attract an outstanding variety of leading investigators from around the world working on all of the major aspects of this topic, and no summary can do justice to the wealth of information presented or the quality of the scientific interactions that took place.
It is a particular pleasure for me to both attend and summarize this particular conference, as it is organized and run by pulmonologists, and I am a medical oncologist. The fact that this conference focuses on lung cancer signifies an era of increasing awareness and collaboration between pulmonary medicine and the ontology specialties that is desperately important if we are to make real progress in fighting this disease. This has not always been the case.
There are many obstacles to overcome in order to make progress in reducing the morbidity and mortality attributable to lung cancer, and only some of them are scientific. Others are sociological or political. For example, there has been the perception that patients with lung cancer are to blame for their disease, so it is not important to try to cure or prevent it. However, over half of patients with lung cancer diagnosed today are never smokers or ex-smokers, and I strongly feel that even smokers do not "deserve" lung cancer. The opinion that there are no effective therapies is also being chipped away with the advent of new drugs such as Iressa (gefitnib) and Alimta (pemetrexed), and a positive adjuvant chemotherapy trial al this year's American Society of Clinical Oncology. The lack of a powerful advocacy group has also led to a relative understanding for lung cancer research, with no Department of Defense grants, and only approximately one tenth the funding of breast cancer per cancer death. The fact that many states use their multimillion-dollar tobacco settlements to balance their budgets and not to combat tobacco-related disease is also a travesty of justice.
My particular enthusiasm for this year's conference relates to the fact that there has also been a historical lack of interest in lung cancer by some pulmonologists, the specialty usually responsible for its diagnosis. The quality and progress reflected by this conference is evidence that these historical barriers to progress are being overcome. I will thus attempt to summarize the highlights of this meeting from the perspective of a researcher and a lung cancer medical oncologist.
CONFERENCE THEMES
The central theme of this year's Aspen conference was crystallized by Wilbur Franklin, who proposed the concept of premalignancy as the disease and cancer as only the end point. It is clear that unless completely surgically resectable, lung cancers are very hard to treat, and thus we need to focus on prevention and earlier intervention, the focus of this conference. I hope that eventual understanding of the genetic and environmental basis of lung cancers will allow us to identify high-risk populations and to develop effective prevention, early detection, and chemoprevention strategies.
A secondary theme for this conference for me was not just the increasing recognition of the complexity of lung cancers, preneoplasia, and populations at risk, but of the availability of emerging technologies capable of grappling with that complexity. Not all lung cancers are the same; every individual's genetic background and individual environmental exposures factor into their personal risk of acquiring lung cancer. Beyond recognizing these facts that have long been clinically suspected, we now have data on the mechanisms of this heterogeneity, and most importantly the tools to begin to quantify and understand what is going on.
INHERITED RISK MODIFIERS
Ann Schwartz described her efforts to identify genes associated with lung cancer risk through the Genetic Epidemiology of Lung Cancer Consortium. To date, this consortium has identified approximately 40 families of a planned 85 with a familial clustering of cases. She emphasized the distinction between the genetics of a single, low-prevalence, high-penetrance gene and a high-prevalence, low-penetrance susceptibility gene. Their data suggest that such a single gene may exist, and the families being collected by this consortium should help map the location of this putative gene. The relative risks for family members of a ease range from 1.3 to 4.8 for all cases, or up to 6.1 for family members of young nonsmokers with lung cancer, all significant levels of risk that justify vigorous pursuit of the putative genes. (1)
Both Dr. Schwartz and Jonathan Samet reviewed the data on susceptibility genes in lung cancer. The genes analyzed to date are primarily those associated with variability in the handling of environmental carcinogens, including phase I, phase II, and DNA repair genes. Single genes tended to be associated with risks of 1.3 to 2.0, but the combination of CYP1A1 with GSTM1 had an overall risk of 3.0 to 5.8. Dr. Schwartz reported on a population study in nonsmoking and early-onset disease based on the Detroit Surveillance, Epidemiology, and End Results registry. This study involved nonsmokers aged 40 to 84 years, and has identified 325 cases and 141 biological samples. Also identified were those with early onset disease appearing at age < 40 years, with 641 cases and 372 samples obtained. There were 181 eases of early onset disease in nonsmokers. The strongest risk identified to date was for CYP1B1 with an odds ratio of 5.5 (CI, 3.4 to 9.1) in nonsmokers. This gene is involved in metabolism of polycyclic aromatic hydrocarbons and estrogen metabolism. It is perhaps counterintuitive, however, that a phase I enzyme association should be found to strongest in nonsmokers, as it is thought to be important in handling the carcinogens associated with cigarette smoking. One possible interpretation is that high doses of carcinogens from smoke overwhelm inherited variability in the phase I enzymes, and that these are manifest only with lower-level exposures that occur in nonsmokers.
It was pointed out by Dr. Samet that since these studies all adjust for tobacco exposure, it is also possible that genetic susceptibility to nicotine addiction would not be detected in these studies. There are data, in fact, suggesting a linkage between dopamine receptor polymorphisms and tobacco use. These sorts of associations should be further explored.
If the observed associations with these genes hold up in larger studies, they could have individual clinical predictive significance, but it is clear that risk associations in smokers may involve whole pathways of genes rather than single genes due to the complexity of carcinogens in tobacco smoke and their various metabolic pathways. In the future, use of bioprobes for entire pathways or comprehensive single nucleotide polymorphism analysis of large numbers of genetic polymorphisms may be useful for more accurately estimating individual risks.
MECHANISMS OF GENETIC ALTERATION
Dr. Samet reviewed the history of the evidence linking environmental factors to lung cancer. While there were many who suspected a link much earlier, solid data associating smoking with lung cancer started with the studies of Ernst Wynder, Morton Levin, and Richard Doll in the 1950s. The links to radon, asbestos, and atmospheric factors such as diesel exhaust were also reviewed. Dr. Samet emphasized the concept of joint causation--the fact that individual cases might be caused by a combination of genetic and environmental factors. Thus, if a single factor is associated with 10% of the cases, elimination of this cause will not cause a 10% drop in incidence, but something less (the concept that "100 minus 10 is not 90"). The role of passive smoking is still somewhat controversial, but most studies place the risk at approximately twofold to fourfold.
The changing proportion of adenocarcinoma among all non-small cell lung cancers is an unexplained, but potentially important observation that could reveal underlying mechanisms and candidates for cancer control strategies. Hampering the study of this phenomenon is the lack of good animal models for nonadenocarcinoma models for lung cancer. Possibilities include the increased use of filtered cigarettes with a concomitant decrease in average particle size and site of airway deposition. Also raised was the introduction of "ammonia technology" in the 1960s to produce a product with a stronger nicotine effect. This may have caused a transition from benzpyrene to nitrosamine predominance in smoke, and thus altered the mutation profiles as well as perhaps the biological consequences of this damage. The data for temporal changes in genetic mutations corresponding to the observed changes in clinical phenotypes is lacking and in need of further investigation.
Provocative data relevant to the identification of the dominant carcinogen in tobacco smoke were presented by Hanspeter Witschi in a smoking A strain mouse model. (2,3) These animals get lung tumors when exposed to chronic tobacco smoke. Filtering the tobacco smoke that dramatically reduces both BAP and nitrosamine content curiously has no effect on the number or type of tumors in this model, which are all adenocarcinomas in either ease. In fact, when pure NNK or BAP are used, many specific chemopreventive agents (such as PITC or d-limonene) reduced the number of tumors observed, but these agents were unexpectedly completely ineffective at reducing the number of tumors resulting from cigarette smoke. These data imply that BAP and NNK may not account for all (or even the majority) of the carcinogenic activity in cigarette smoke, increasing the complexity and complicating the analysis of laboratory carcinogenesis studies. An interesting and unexplained observation in this model is the fact that the tumor number is increased when smoking exposure is followed by a period of dean air exposure. This effect was very clear in that mice killed after continuous smoking exposure had fewer tumors than mice killed after being allowed several months of clean air after smoke exposure. It is thus possible that smoke both induces the necessary changes and restrains the manifestation of tumors in this model.
Thomas Slaga discussed the use of skin cancer as a model for lung tumorigenesis, and reviewed the data strongly linking obesity with cancer. It was pointed out, however, that in the largest study of the linkage between obesity and cancer published to date in recent issue of the New England Journal of Medicine, (4) there was no association with lung cancer. Again, this may be related to the fact that smoking is such a strong risk factor that it overwhelms more modest ones. He also described the potent chemoprotective effect of glucocorticoids in several models of carcinogenesis, including the smoking mouse model. Confirmation of this in human epidemiologic studies is complicated by the fact that the population of patients with COPD is both at highest risk and most likely to use inhaled steroids for symptom control.
Curt Harris discussed the central importance of the p53 pathway in oncogenesis and emphasized the importance of "molecular archeology"--that identification of somatic alterations of specific genes in tumors reveals important biochemical pathways and mechanisms, and that the types of mutations that occur in these genes call shed light on the mutational mechanisms involved. One example is the fact that lung cancers typically have a predominance of G to T transversions compared to the G to A transitions commonly observed in colon cancer, thought to be related to the differences in environmental etiology. (5) He highlighted a recent study (6) showing that in lung tumors in which the MGMT gent (involved in the repair of methyl guanine) is silenced by methylation, an increased number of G to A mutations is observed. The biology underlying the clear differences in the codon location of mutations in p53 is less well understood, but may be primarily "chemical"--differences in the local sequence affect benzo-apyrene adduct formation vs CpG methylation.
Both Drs. Gazdar and Herman described nonmutation-based mechanisms tumors utilize for the silencing of tumor-suppressor genes involving promoter methylation. This is clearly now a validated mechanism confirmed by several groups. Data were presented that multiple genes important in oncogenesis including p16, MGMT, and RAR[beta] were frequently methylated and thus transcriptionally silenced. The underlying alterations that cause these changes in methylation are as yet unclear and may ultimately be better intervention targets than global demethylation strategies.
DETECTING EARLY CANCER AND PREMALIGNANT STATES
The traditional methods for detecting premalignant changes involve bronchoscopy with light microscopic evaluation of biopsy specimens. Strong data from many studies have underscored the relationship of high grades of dysplasia with increased risk of lung cancer. Increased detection sensitivity can be achieved using a fluorescence bronchoscope that detects changes in tissue autofluorescence associated with preneoplasia. Wilbur Franklin presented data from the Colorado Lung Specialized Program of Research Excellence (SPORE) effort with 11,000 patients enrolled on the basis of cigarette smoking history, obstructive lung disease, and sputum dysplasia. In this study, approximately 50% had mild dysplasia on bronchoscopic biopsy, 22% had moderate dysplasia, and 1.3% were found to have invasive cancer. This emphasizes that these characteristics accurately identify an extremely high-risk group of patients with a cancer incidence rate approaching that of the helical CT screening trials.
However, while these criteria identify a population at risk, the individual predictive value of light microscopic changes is not ideal. Jeremy George, Middlesex, UK, presented intriguing data on this point. (7) He presented a surveillance study of "premalignancy" observed by light microscopy of bronchoscopic biopsy specimens. He found that over time some of the severe dysplasias regressed, while some progressed. Cancers developed in some of these sites of severe dysplasia, but not others. Most interesting, several cancers developed in sites within the lung with a normal appearance by white light and Laser Induced Fluorescent Endoscopy bronchoscopy, and also normal by light microscopy. He concluded that the airways in these patients are extremely unstable. This is also evidence that the canonical sequence of metaplasia--dysplasia--carcinoma in situ--invasive cancer is not as linear as initially thought. It is dear that light microscopically observable preneoplastic alterations of bronchial epithelial cells precede only a relatively small fraction of clinical cancers. It is in fact difficult to find adjacent dysplasia in resection specimens. These abnormalities also sometimes regress spontaneously. In addition, while these lesions are highly associated with squamous cell carcinoma (a decreasing subset of non-small cell lung cancer), they are less so for adenocarcinoma or small cell lung cancer, the latter disease also highly smoking related. I feel that smoking causes both dysplasia and calmer, but that these are "true, true, and only partially related." The most informative investigation would be to study underlying genetic alterations in bronchial epithelial cells independent of light microscopic changes (perhaps, however, focusing on patient populations identified as being at high risk by virtue of the presence of these light microscopic changes). Thus, there should be an increasing emphasis on molecular methods for lung cancer early detection or risk evaluation "unbiased" by light microscopy.
MOLECULAR SCREENING
Since it is thought that cancer is the result of an accumulation of genetic alterations resulting in protein dysregulation (up or down), most approaches to the molecular early detection of cancer or premalignancy rely on overexpression or underexpression of proteins or the detection of significant genetic alterations in biosamples. Wilbur Franklin presented data on biomarkers of preneoplasia including vascular endothelial growth factor, Ki67, and RAR[beta] overexpression by immunohistochemistry, and methylation markers performed with Steve Belinsky. (8) In these studies, they took advantage of a nested subset of the samples from the Colorado SPORE of cases who acquired lung cancer and matched control subjects. These data presented increased overall risks of lung cancer up to 13.9-fold for combinations of methylation markers, but with a high false-positive rate. He also presented his own microarray data that has discovered frequent high-level overexpression of cancer testis antigens in lung cancer. Pierre Massion discussed new markers that he discovered within a region of chromosome 3q frequently amplified in squamous cell lung cancers. (9) This amplicon contains the p63 gene, and is amplified and overexpressed in most squamous cell tumors as well as in severe dysplasia and carcinoma-in situ. Also in this amplicon are the RNA component of telomerase and the phosphoinositol-3 kinase catalytic subunit. It was brought up in the discussion that sophisticated multimarker "cluster analysis" similar to that being done with microarrays should be attempted for these sorts of markers and may be more predictive in individual cases than any single marker or simple combinations.
Along these lines, I presented work from my laboratory that identified patterns of proteins detected by mass spectrometry, associated with lung cancer and its subtypes, as well as biological behaviors such as nodal involvement and survival. The concept of thinking in multigene modules was also emphasized by Naftali Kaminski, who presented complementary DNA microarray data from the University of Pittsburgh using advanced statistical methods and a training-testing design. He introduced the concept of "in silico microdissection," in which patterns associated with various cell types (such as stromal, hematopoietic, or vascular cells) could be teased out of array data from bulk tumor samples. These analyses could be useful for dissecting important tumor-host interactions.
Dr. Kaminski also presented intriguing data that gene expression patterns of peripheral blood mononuclear cells of smokers with cancer could be distinguished from smokers without cancer on array analysis. He discussed the proteomics approaches to serum detection pioneered by Petricoin et al, and contrasted the array approach. The richness of the data obtained is much greater with the complementary DNA array approach, but Dr. Kaminski hypothesized that ultimately clinically useful screening tools will likely be based on protein markers.
RADIOGRAPHIC SCREENING
The traditional methods for detecting lung cancer are primarily radiographic, and Dr. Jett reviewed the status of the current studies looking at the utility of screening high-resolution CT scans. With the advent of helical multidetector scanners, these studies are yielding a wealth of data on the incidence, types, and natural history of tiny lesions in the lung. Data from Mayo Clinic (10) demonstrate that a significant number of nodules are detected; in fact, approximately 70% of screened subjects had a noncalcified nodule. Approximately 2% of the patients were found to have either prevalent or incident tumors after three serial scans. Swenson et al (10) presented data that tumors of all histologies were detected, including tumors at other sites within the scanned region of the body. The large ongoing national randomized trial (National Lung Screening Trial) should help answer the question about the clinical impact of this screening method, but it also generates a wealth of information on the biology of lung cancer, and importantly provides a large population of patients for molecular studies.
CHEMOPREVENTION
The history of chemoprevention approaches for lung cancer was reviewed lay Gil Omenn from the University of Michigan. The major studies were the [alpha]-Tocopherol, Beta-Carotene Cancer Prevention Study (11) and the BetaCarotene and Retinol Efficacy Trial, (12) involving tens of thousands of subjects, and they both found increases in the incidence of lung cancer in the arms administered betacarotene, with an approximate 1.3-fold increased risk, with about 59 cases vs 46 cases per 10,000 person-years. The pilot trials in progress were also reviewed, including those using celecoxib, 5-LOX inhibitors, and other novel targets. In his summary, Dr. Omenn emphasized several points that bear repeating: (1) the importance of pushing new agents into clinical trials because the potential benefit is great; (2) lung cancers are heterogeneous, and agents may affect one subset and not another; (3) pathways found to be effective in chemopreventing other cancers should be tested in lung cancer (eg, nonsteroidal anti-inflammatory drugs with colon and signal transduction targets in breast); (4) consideration of targeting inflammatory or stromal components; (5) continued development of surrogate markers and end points; (6) recognition that all agents have multiple effects, positive and negative; and (7) consider the use of proven therapy drugs in chemoprevention (eg, Iressa).
The role of cyclooxygenase-2 (COX-2) in lung cancer was another emphasis at this meeting, with many investigators presenting data from University of Texas Southwestern, University of Colorado, University of California Los Angeles (UCLA) and Vanderbilt. While studies are underway evaluating the effect of nonsteroidal anti-inflammatory drag use on lung cancer incidence and at least one celecoxib chemoprevention trial is open, the theme presented at this meeting was the complexity and sometimes-opposite effects of COX-2 products in lung cancer. Dr. Gazdar pointed out that while COX-2 is overexpressed in many non-small cell lung cancers (especially adenocarcinomas), it has a lower expression in small cell lung cancer than normal lung, raising the possibility that inhibitors will have differential effects on these two basic histologies. COX-2 inhibitors may also have differential effects on adenocarcinoma vs squamous cell carcinoma. The COX-2 system, as many others presented at this conference, is also complicated by the multitude of metabolites downstream of cyclooxygenase. Data were presented that while prostacyclins and prostaglandins are both COX-2 products, prostacyclins seem to inhibit cancer while prostaglandins (13) and thromboxanes seem to promote cancer. Mark Geraci and Patrick Nana-Sinkam at the University of Colorado found that a surfactant protein C promoter-driven prostacyclin synthase transgenic mouse is resistant to carcinogen induced lung cancer (14) and that human lung cancers are high in prostaglandin [E.sub.2] but low in prostacyclin, the opposite of that observed in normal lung. It thus may make more sense to target more distal pathways such as using a combination of a prostaglandin antagonist with a prostacyclin agonist. Iloprost is a prostacyclin agonist being tested in a randomized chemoprevention trial at University of Colorado based on these observations, and enrolls smokers with sputum atypia. The end points for this trial are dysplasia index and Ki67-staining surrogate markers.
NOVEL TARGETS
Multiple interesting potential therapy/prevention targets were discussed in this meeting, which space does not allow me to describe. These include STAT and Smad regulators, hemidesmosome components, and TNF-related apoptosis-inducing ligand receptors. Drs. Dennis and Kurie both emphasized the importance of the phosphoinositol kinase and protein kinase C pathways in lung cancer. These pathways are activated by growth factors, and are associated with resistance to chemotherapy and radiation. Dr. Dennis presented interesting data that nicotine alone can induce the expression and activation of epidermal growth factor receptor. This has implications in both the development of lung cancer and perhaps the observed relative resistance of tumors arising in smokers to EGFr tyrosine kinase inhibitors. Jill Siegfried presented very interesting data on the prognostic impact of the HGF/cMet pathway and the in vitro effects of human growth factor inhibition on tumor cell line growth. Michael Keane from UCLA presented data that the chemokine receptor CXCR2 knockout mice have reduced tumor growth and angiogenesis, and that anti-CXCR2 antibodies slow growth and prolong survival in both heterotopic and orthotopic mouse models. John Stewart from the University of Colorado presented his work on bradykinin antagonists, which have resulted in several peptide and nonpeptide antagonists with potent antitumor effects in mouse models. One of these is being produced for clinical trial through the Rapid Access to Intervention Development contract program.
CONCLUSIONS
In contrast to the state of the art 10 to 15 years ago, there are now convincing data that an impact is being made on the morbidity and mortality inflicted by lung cancer. We have an increasing amount of data and clinical acceptance that therapy can prolong survival and improve the quality of life of patients with advanced lung cancer, and can prolong the survival of resected patients. The first signal transduction inhibitor approved for lung cancer, Iressa (gefitinib), has now shown real activity, in chemotherapy refractory tumors. In spite of these glimmers of hope, the magnitude of the problem of advanced lung cancer is still daunting, and this has spurred interest in prevention, early detection, and early interventions, the themes of this year's Aspen Conference. These issues represent the interface between pulmonary medicine and oncology specialties, and this conference has been of great value in strengthening this association. Array technologies, biomarker development, and proteomics are sure to make an impact in the next decades, and slow progress is being made on lung cancer chemoprevention. We at least know what does not work. The problem of lung cancer needs greater public awareness and increased patient advocacy to achieve deserved parity with other, less common causes of cancer death that are relatively better funded, and appropriate use of the tobacco settlement funds for research and smoking cessation/prevention is essential.
REFERENCES
(1) Schwartz AG, Rothrock M, Yang P, et al. Increased cancer risk among relatives of nonsmoking lung cancer cases. Genet Epidemiol 1999; 17:1-15
(2) Witschi H, Espiritu I, Dance ST, et al. A mouse lung tumor model of tobacco smoke carcinogenesis. Toxicol Sci 2002; 68:322-330
(3) Witschi H. Successful and not so successful chemoprevention of tobacco smoke-induced lung tumors. Exp Lung Res 2000; 26:743-755
(4) Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort, of U. S. adults N Engl J Med 2003; 348:1625-1638
(5) Chiba I, Takahashi T, Nau MM, et al. Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Oncogene 1990; 5:1693-1610
(6) Wolf P, Hu YC, Doffek K, et al. O(6)-Methylguanine DNA methyltransferase promoter hypermethylation shifts the p53 mutational spectrum in non-small cell lung cancer. Cancer Res 2001; 61:8113-8117
(7) Banerjee AK, Rabbitts PH, George J. Lung cancer: 3. Fluorescence bronchoscopy; clinical dilemmas and research opportunities. Thorax 2003; 58:266-271
(8) Belinsky SA, Palmisano WA, Gilliland FD, et al. Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer Res 2002; 62: 2370-2377
(9) Massion PP, Kuo WL, Stokoe D, et al. Genomie copy number analysis of non-small cell lung cancer using array comparative genomic hybridization: implications of the phosphatidylinositol 3-kinase pathway. Cancer Res 2002; 62:3636-3640
(10) Swensen SJ, Jett JR, Hartman TE, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology 2003; 226:756-761
(11) Albanes D, Heinonen OP, Taylor PR, et al. Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: effects of base-line characteristics and study compliance. J Natl Cancer Inst 1996; 88:1560-1579
(12) Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst 1996; 88:1550-1559
(13) Yang L, Yamagata N, Yadav R., et al. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest 2003; 111:727-735
(14) Keith RL, Miller YE, Hoshikawa Y, et al. Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res 2002; 62:734-740
* From the Experimental Therapeutics Program, Vanderbilt-Ingrain Comprehensive Cancer Center, Nashville, TN.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (e-mail: permissions@chestnet.org).
Correspondence to: David P. Carbon, MD, PhD, Experimental Therapeutics Program, Vanderbilt-Ingram Comprehensive Cancer Center, 648 Preston Rsch Bldg, Nashville, TN 37232-6838
COPYRIGHT 2004 American College of Chest Physicians
COPYRIGHT 2004 Gale Group