Business Editors/Health/Medical Writers
CHICAGO--(BUSINESS WIRE)--June 1, 2003
In a head-to-head trial comparing Alimta(R) (pemetrexed) to Taxotere(R) (docetaxel), Alimta showed a similar survival benefit with significantly fewer side effects in patients with recurrent non-small cell lung cancer (NSCLC). Results of this Phase III trial -- the largest Phase III trial ever conducted in second-line NSCLC -- were presented today at the 39th annual meeting of the American Society of Clinical Oncology, which is being held in Chicago.
"Before Taxotere, we had no agent that was proven to prolong life for patients with recurrent non-small cell lung cancer," said Nasser Hanna, M.D., who presented the findings and is an assistant professor of medicine at Indiana University Cancer Center. "Now, with these data on Alimta, we have observed a survival advantage, just as we have with Taxotere, but with fewer side effects."
Today's announcement was based on a global study of 571 patients with NSCLC previously treated with chemotherapy. Of these patients, 283 were randomized to receive Taxotere (75 mg/m2 on day one of a 21-day cycle; one-hour infusion). The remaining 288 patients received Alimta (500 mg/m2 on day one of a 21-day cycle; 10-minute infusion) supplemented with vitamin B12 and folic acid. Unlike Alimta, which is an investigational agent, Taxotere is approved for use in the treatment of first- and second-line NSCLC in both the U.S. and Europe.
According to the results:
-- Median survival, the study's primary endpoint, was similar
between the two study arms. Those receiving Alimta had a
median survival of 8.3 months, which means that half of the
patients survived longer than 8.3 months and half shorter. The
median survival for those who received Taxotere in this study
was 7.9 months.
-- The incidence of severe neutropenia, a decrease in the number
of white blood cells that increases the risk of infection, was
5 percent in the Alimta arm and 40 percent in the Taxotere
arm, a difference that was statistically significant
(p= less than 0.001).
-- The difference in the incidence of neutropenic fever and
subsequent hospitalizations between the Alimta and Taxotere
arms was also statistically significant: 2 percent for the
Alimta patients compared to 13 percent for the Taxotere
patients (p= less than 0.001).
-- In a third finding that reached statistical significance, the
incidence of drug-related serious adverse events -- which
include side effects that could lead to a life-threatening
outcome, death or hospitalization -- was 10 percent for Alimta
patients and 24 percent for Taxotere patients
(p= less than 0.001).
-- The incidence of grades 3/4 Alanine Transaminase (Alanine
Transaminase, or ALT, is a laboratory measurement of liver
function) was 1.9 percent in the Alimta arm, a rate that
was significantly greater than in the Taxotere arm (p=0.028).
According to the findings, the incidence of grades 3/4 ALT in
the Alimta arm was transient.
"Patients with cancer who are being treated in the second-line setting must heavily consider the risk-benefit ratio of another course of chemotherapy. It can be a difficult balancing act, but in this study, patients who received Alimta retained the chance of a benefit, but with fewer side effects, and that is important," said Paolo Paoletti, M.D., vice president of oncology products at Eli Lilly and Company. He added that Lilly is also evaluating the activity of Alimta in the first-line treatment of NSCLC.
Alimta in First-Line NSCLC
At this year's ASCO meeting, the following abstracts are being presented involving the use of Alimta in the first-line treatment of advanced NSCLC:
-- Alimta plus carboplatin in patients with advanced non-small
cell lung cancer: A phase II trial (Abstract #2580)
-- Phase II randomized study of pemetrexed + carboplatin or
oxaliplatin, as front-line chemotherapy in patients with
locally advanced or metastatic non-small cell lung cancer
(Abstract #2513)
"Alimta has already shown promising activity when combined with cisplatin in malignant pleural mesothelioma, a difficult-to-treat cancer in the lining of the lungs, and today's data support use with platinum compounds for non-small cell lung cancer," said Christian Manegold, professor of medicine at the University of Heidelberg, and consultant for medical oncology at the Thoracic Hospital in Heidelberg, Germany. "These findings are especially meaningful in Europe where platinum regimens provide feasible alternatives so patients may receive treatment on an outpatient basis. With their ease of use, Alimta combinations could provide attractive and convenient choices for these patients suffering from non-small cell lung cancer."
Lilly, a leading innovation-driven corporation is developing a growing portfolio of best-in-class pharmaceutical products by applying the latest research from its own worldwide laboratories and from collaborations with eminent scientific organizations. Headquartered in Indianapolis, Ind., Lilly provides answers -- through medicines and information -- for some of the world's most urgent medical needs.
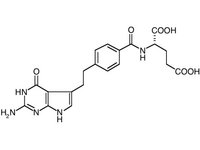
Alimta(R) (pemetrexed, Lilly)
Taxotere(R) (docetaxol, Aventis Pharmaceuticals, Inc.)
COPYRIGHT 2003 Business Wire
COPYRIGHT 2003 Gale Group