Roentgenographic manifestations of two cases of pulmonary involvement with myeloma were presented. One showed a nodular mass lesion extending from an extramedullary mediastinal plasmacytoma into the lung parenchyma while the other showed diffuse reticulonodular infiltrate by myeloma cells in association with alveolar septal amyloidosis. These two cases demonstrate the extreme variability of pulmonary involvement by myeloma, which can mimic a solitary tumor or an inflammatory infiltrate.
(Chest 1992; 102:946-48)
Extramedullary myeloma usually involves the liver, spleen, and lymph nodes.[1] Involvement of pulmonary parenchyma by myeloma cells either as a plasmacytoma or as a pulmonary infiltrate is rare.[1-8] No symptoms or physical findings referable to the respiratory tract were mentioned in one series of 57 cases of myeloma reported by Kenny and Moloney[2] and another series of 869 cases reviewed by Kyle.[8] Reported herein are the cases of two patients, one with extramedullary plasmacytoma involving the mediastinum and lung while another was manifested by diffuse pulmonary infiltrate by myeloma cells accompanied by amyloid deposition. The former behaved as a solid tumor and the latter mimicked diffuse inflammatory infiltrate.
Case Reports
Case 1
A 74-year-old previously healthy man developed some wheezing and vague chest discomfort for about ten days and had a routine chest roentgenogram taken that showed a well-demarcated opacity in the right infrahilar region encroaching on and circumferentially enveloping the right middle and lower lobe bronchi (Fig 1). Findings from the review of his systems were otherwise essentially unremarkable. A bronchoscopy revealed that the right main-stem bronchus appeared to be involved by a mass lesion. Thoracic computed tomography showed a large mass in the posterior mediastinum extending from the right clavicle down to the diaphragm and also to the right hilum with encasement of the right main-stem and lower lobe bronchi (Fig 2). Mediastinal lymphadenopathy was also present. Skeletal survey failed to demonstrate osteolytic lesions in all portions of the axial skeleton and proximal long bones. Brain and liver-spleen scans were all normal. Biopsy specimens of the posterior mediastinal mass lesion and fine needle aspirate of the tumor in the right lung all revealed numerous monomorphic mature plasma cells that selectively stained positive for IgG and lambda light chain but negative for other immunoglobulins and kappa light chain. Paraprotein was, however, not detected in the plasma and urine, and bone marrow aspirate contained only 1 percent plasma cells. Mediastinal plasmacytoma with lung involvement was thus diagnosed.
The patient subsequently received radiation therapy (5,000 rad in 28 fractions) with remarkable dissolution of the mass lesion. Repeated bone marrow aspirate and plasma protein electrophoresis showed no evidence of myeloma. The patient remained in remission until five years later when recurrent plasmacytoma appeared in the stomach. The patient is now receiving chemotherapy.
Case 2
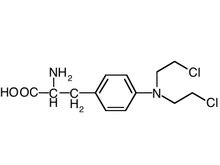
A 68-year-old woman was diagnosed as having IgA kappa light chain extramedullary plasmacytoma from a forearm biopsy 12 years ago. She was treated intermittently with melphalan (Alkeran), prednisone, and radiation therapy. The disease had run a fairly indolent course. She had been receiving no therapy for the last eight months but was readmitted to the hospital because of chronic cough and worsening of dyspnea for several months. Pulmonary function tests showed a mixed restrictive and obstructive defect. Chest roentgenography revealed reticulonodular opacities in lower lobes of both lungs with confluent nodular opacities in both lung bases. Transbronchial biopsy specimens at that time showed organizing pneumonia and bronchial washings grew Nocardia. She was treated with steroids and sulfa drugs with some improvement. However, in the ensuing months, cough and dyspnea recurred, and chest roentgenographic findings worsened. Reticulonodular opacities were seen throughout both lungs with markedly increased opacities in the bases (Fig 3). She underwent a bronchoscopy with transbronchial biopsy specimens that were nondiagnostic and subsequently she had an open lung biopsy. A firm nodule was found in the lung specimen, which was composed of numerous plasma cells. Away from the nodule, there were interstitial infiltrates by mononuclear cells, including plasma cells and deposition of amorphous eosinophilic material around and in the media of blood vessels that were stained positive for amyloid with Congo red. Bone marrow aspirate showed 10 percent plasma cells and serum IgA was 3,900 mg/dl. The patient was initially treated with melphalan and prednisone with no improvement. She was then treated with vincristine, doxorubicin (Adriamycin), and dexamethasone (Decadron).
Discussion
Pulmonary involvement in myeloma is so rare that no attention has been paid to such occurrence in several large series.[2,8] Kintzer et al[9] found that 46 percent of patients in a series of 958 cases had thoracic involvement by myeloma. Most of them showed bone involvement or pulmonary infiltrate secondary to an infectious process. Only 11 patients developed extramedullary plasmacytoma in the thorax, and four patients had pulmonary infiltrate suggestive of myeloma cell infiltrate (only one proven). Pulmonary involvement seems to be more commonly associated with the aggressive terminal phase of myeloma[10,11] or plasma cell leukemia.[12] Rare cases of primary pulmonary plasmacytoma have been reported,[5,6] and the first case of tracheobronchial involvement by myeloma tissue was described recently.[13]
The present two cases represent two types of myeloma involvement of the lung, namely, nodular mass lesion and diffuse reticulonodular pulmonary infiltrate. The mass lesion in the right lung in case 1 apparently extended from a larger mass in the posterior mediastinum instead of being a primary pulmonary plasmacytoma. Such an occurrence is a rule rather than an exception because either myeloma or solitary plasmacytoma of the lung parenchyma is extremely rare.[5,6] The presence of air bronchograms in this patient indicated that the malignant plasma cells infiltrated diffusely the lung parenchyma without total occlusion of bronchial lumens.
Diffuse pulmonary infiltrate by myeloma cells as demonstrated in case 2 is very rare.[14-16] Such infiltration in our patient was accomplished by amyloid deposition, particularly around the blood vessels, so-called alveolar septal amyloidosis.[15,16] Although direct myeloma cell infiltration of the lung is uncommon, the lungs are involved in 36 to 92 percent of patients with primary systemic amyloidosis.[17,18] The lesions are usually confined to the tracheobronchial tree and interstitial tissues; on rare occasions they extend along the alveolar septa and the media of the blood vessels as in case 2. Since this patient had only about 10 percent plasma cells in her bone marrow but had an IgA peak of about 4 g/dl, it was thought by the attending hematologist that this was likely due to the production of IgA by the plasma cells in her lungs. Indeed, a recent case study provides evidence indicating an ongoing pulmonary immune response resulting in excess paraprotein in the pulmonary compartment leading to pulmonary amyloid deposit.[16]
The above two cases demonstrate the variability of roentgenographic manifestations of pulmonary involvement by myeloma. In case 1 the tumor differentiated from other solid mediastinal tumors while in case 2 the infiltrate differentiated from diffuse pulmonary inflammatory processes.
References
[1] Gabriel S. Multiple myeloma presenting as pulmonary infiltration: report of a case. Dis Chest 1965; 47:123-26
[2] Kenny JJ, Moloney WC. Multiple myeloma: diagnosis and management in a series of 57 cases. Ann Intern Med 1957; 46:1079-91
[3] Favis EA, Kerman JD, Schildecker W. Multiple myeloma manifested as a problem in the diagnosis of pulmonary disease. Am J Med 1960; 28:323-27
[4] Freeman Z. Myelomatosis with extensive pulmonary involvement. Thorax 1961; 16:378-81
[5] Sekulich M, Pandola G, Simon T. A solitary pulmonary mass in multiple myeloma: report of a case. Dis Chest 1965; 48:100-03
[6] Mazumdar P, Abraham S, Damodaran VN, Saha MC. Pulmonary plasmacytoma. Am Rev Respir Dis 1969; 100:866-69
[7] Badrinas F, Rodriquez-Roisin R, Picado C. Multiple myeloma with pleura involvement. Am Rev Respir Dis 1969; 110:82-7
[8] Kyle RA. Multiple myeloma: review of 869 cases. Mayo Clin Proc 1975; 50:29-40
[9] Kintzer JS, Rosenow EC, Kyle RA. Thoracic and pulmonary abnormalities in multiple myeloma: a review of 958 cases. Arch Intern Med 1978; 138:727-30
[10] Suchman AL, Coleman M, Mouradian JA, Wolf DJ, Saletan S. Aggressive plasma cell myeloma: a terminal phase. Arch Intern Med 1981; 141:1315-20
[11] Garewal M, Durie BGM. Aggressive phase of multiple myeloma with pulmonary plasma cell infiltrates. JAMA 1982; 248:1875-76
[12] Case 20-1987, Case records of the Massachusetts General Hospital: Weekly clinicopathological exercises. N Engl J Med 1987; 316:1259-67
[13] Gilchrist D, Chan CK, LaRoye GJ, Messner HA, Curtis JE. Bronchial mucosal infiltrate and unilateral lung collapse: an unusual complication of multiple myeloma. Am J Med 1988; 85:741
[14] Thompson PJ, Citron KM. Amyloid and the lower respiratory tract. Thorax 1983; 38:84-7
[15] Poh SC, Tjia TS, Seah HC. Primary diffuse alveolar septal amyloidosis. Thorax 1975; 30:186-91
[16] Morgan JE, McCaul DS, Rodriguez FH, Abernathy DA, deShazo RD, Banks DE. Pulmonary immunologic features of alveolar septal amyloidosis associated with multiple myeloma. Chest 1987; 92:704-08
[17] Duff GL, Murray EGD. Primary systemic amyloidosis. Am J Med Sci 1954; 328:317-33
[18] Celli BR, Robinow A, Cohen AS, Brody JS. Patterns of pulmonary involvement in systemic amyloidosis. Chest 1978; 74:543-47
COPYRIGHT 1992 American College of Chest Physicians
COPYRIGHT 2004 Gale Group