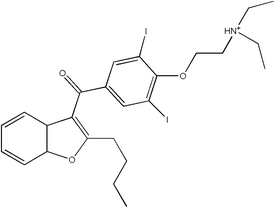
Amiodarone (Cordarone) is a complex antiarrhythmic agent with multiple electrophysiologic effects, unusual pharmacokinetics, and numerous potentially harmful drug interactions and adverse effects. Although the U.S. Food and Drug Administration (FDA) has labeled amiodarone only for the treatment of life-threatening ventricular arrhythmias, the drug also is used to treat atrial fibrillation. Because of the complexity and widespread use of this agent, other treatment decisions often are affected. This article reviews the pharmacology, indications, adverse effects, and drug interactions of amiodarone, and outlines a strategy for surveillance of patients who are taking this drug.
Clinical Pharmacology
PHARMACOKINETICS
Amiodarone is an iodine-containing compound with some structural similarity to thyroxine. The drug's high iodine content likely is a factor in its effects on the thyroid gland. The bioavailability of amiodarone is variable but generally poor, ranging from 22 to 95 percent. (1) Absorption is enhanced when the drug is taken with food. (2) Amiodarone is highly lipid soluble and is stored in high concentrations in fat and muscle, as well as in the liver, lungs, and skin. Amiodarone crosses the placenta and reaches measurable levels in breast milk.
The major metabolite of amiodarone is desethylamiodarone (DEA), which is known to have antiarrhythmic properties. Grapefruit juice can inhibit amiodarone metabolism and lead to elevated drug levels,3 but the impact of this interaction on the long-term efficacy and toxicity of amiodarone is not known.
The elimination half-life of amiodarone is highly variable and unusually long, averaging about 58 days. The long half-life is thought to be a result of the drug's slow release from lipid-rich tissues. (2)
ELECTROPHYSIOLOGIC EFFECTS
Amiodarone is considered to be a class III drug (Vaughan Williams classification), which indicates that it prolongs the QT interval. However, the drug has many other effects: it slows heart rate and atrioventricular nodal conduction (via calcium channel and beta-receptor blockade), prolongs refractoriness (via potassium and sodium channel blockade), and slows intracardiac conduction (via sodium channel blockade).
The relationship between plasma amiodarone concentrations and effect, as well as the contribution of the metabolite DEA, is not well established. (2) Routine monitoring of the amiodarone plasma level is not recommended. (4) [Evidence level C, consensus/ expert guidelines]
Indications
LONG-TERM TREATMENT
Amiodarone is approved for use in the secondary prevention of life-threatening ventricular arrhythmias. The North American Society for Pacing and Electrophysiology (NASPE) recommends amiodarone as the antiarrhythmic agent of choice in patients who have survived sustained ventricular tachyarrhythmias, particularly those with left ventricular dysfunction. (4)
Studies on the use of amiodarone for the primary prevention of sudden death in high-risk patients have had mixed results. One meta-analysis of 13 studies of patients with congestive heart failure or recent myocardial infarction showed a small reduction in total annual mortality, from 12.3 percent to 10.9 percent (absolute risk reduction [ARR], 2.4 percent; number needed to treat [NNT], 42). (5) [Evidence level A, meta-analysis] The benefit of amiodarone therapy was more pronounced in the patients who had congestive heart failure, with treatment reducing the annual mortality rate from 24.3 percent to 19.9 percent (ARR, 4.4 percent; NNT, 23). Because implantable cardioverter-defibrillators (ICDs) are more effective than amiodarone in reducing mortality in high-risk patients with previous myocardial infarction, primary treatment should be an ICD. (6-9) [Reference 6--Evidence level A, meta-analysis] In these patients, amiodarone may be used as an adjunct to reduce the frequency of ICD shocks or to control atrial fibrillation in selected highly symptomatic patients. The relative efficacy of amiodarone and ICDs in preventing sudden death in patients without coronary disease is under investigation.
Amiodarone is used in the treatment of atrial fibrillation, although the FDA has not approved this indication. Various practice guidelines recommend amiodarone as a second-line drug in the long-term treatment of atrial fibrillation in patients with structural heart disease and in highly symptomatic patients without heart disease. (10) Several smaller studies have shown that amiodarone is similar to quinidine and sotalol in the treatment of atrial fibrillation in these patients. (11,12) In one randomized controlled trial (RCT), (12) sinus rhythm was maintained successfully for 16 months in 65 percent of patients treated with amiodarone, compared with 37 percent of patients treated with sotalol or propafenone (ARR, 28 percent; NNT, 3.6). However, recent studies have shown that aggressive attempts to maintain sinus rhythm using amiodarone or other drugs do not improve outcomes in relatively asymptomatic patients. (13,14) Therefore, long-term amiodarone therapy, with its potential for toxicity, does not appear to be justified in patients who are taking anticoagulant drugs if rate-control strategies can provide satisfactory symptomatic improvement.
ACUTE TREATMENT
Intravenously administered amiodarone is effective for the emergency treatment of ventricular tachyarrhythmias. Onset of the antiarrhythmic effect of intravenous amiodarone occurs in less than 30 minutes. (15)
In the Advanced Cardiac Life Support (ACLS) guidelines published in 2000, amiodarone and procainamide are recommended for the initial treatment of hemodynamically stable wide-complex tachycardia. (16) However, these guidelines list amiodarone as being only "possibly effective" for the treatment of refractory pulseless ventricular tachycardia or ventricular fibrillation. In contrast, a recent study comparing the use of amiodarone and lidocaine in patients with shock-resistant, out-of-hospital ventricular fibrillation showed that amiodarone therapy substantially improves survival and hospital admission rates. (17) [Evidence level A, RCT]
Typical amiodarone dosages in the ACLS setting are provided in Table 1. (2,10) In patients who require long-term treatment, intravenous dosing should be switched to oral dosing. Patients who received intravenous amiodarone for less than one week should take 800 to 1,600 mg oral amiodarone per day. (2) Patients who received intravenous amiodarone for one to three weeks should take 600 to 800 oral amiodarone per day, and patients who received intravenous amiodarone for more than three weeks should take 400 mg oral amiodarone per day.
Intravenously administered amiodarone is being used with increasing frequency in the acute treatment of atrial fibrillation. In a meta-analysis of 18 RCTs, amiodarone was similar to other antiarrhythmic drugs in its ability to convert patients to normal sinus rhythm (72.1 percent for amiodarone compared with 71.9 percent for other antiarrhythmic drugs). (18) [Evidence level A, meta-analysis] The meta-analysis did not address the effect of antiarrhythmic drugs on mortality and other clinical outcomes. Use of these drugs would be most appropriate in patients with recurrent hemodynamically unstable atrial fibrillation. (10) Amiodarone may be particularly beneficial in patients with rapid ventricular rates or impaired renal function.
Adverse Effects
Amiodarone has been associated with toxicity involving the lungs, thyroid gland, liver, eyes, skin, and nerves (Table 2). (2,5,11,19) The frequency of most adverse effects is related to the total amiodarone exposure (i.e., dosage and duration of treatment). Therefore, physicians must use the lowest possible dosage of amiodarone and, if possible, discontinue treatment if adverse effects occur.
PULMONARY TOXICITY
The most serious potential adverse effect of amiodarone therapy is pulmonary toxicity, which may result from direct drug-induced phospholipidosis or immune-mediated hypersensitivity. (19) The most common clinical presentation is subacute cough and progressive dyspnea, with associated patchy interstitial infiltrates on chest radiographs and reduced diffusing capacity on pulmonary function tests. A much less common presentation is adult respiratory distress syndrome.
In early studies, the frequency of pulmonary toxicity in amiodarone therapy was 2 to 17 percent. (2) More recent studies have shown a lower incidence in patients receiving dosages of 300 mg per day or less. A meta-analysis11 of double-blind trials found the frequency of adult respiratory distress syndrome to be 1 percent annually.
Routine screening for adult respiratory distress syndrome is of limited value, because pulmonary toxicity can develop rapidly with no antecedent abnormalities on chest radiographs or pulmonary function tests. Any report from the patient of worsening dyspnea or cough should elicit a prompt assessment for pulmonary toxicity. Congestive heart failure can mimic amiodarone pneumonitis and, thus, must be ruled out early in the evaluation. High-resolution computed tomographic scanning can be helpful in making a diagnosis.
The primary treatment for pulmonary toxicity is withdrawal of amiodarone and provision of supportive care and, in some cases, corticosteroids. In most instances, the toxicity is reversible.
THYROID TOXICITY
Thyroid toxicity is the most common complication that requires intervention. Thyroid abnormalities have been described in up to 10 percent of patients receiving long-term amiodarone therapy. (2) Hyperthyroidism may result from an excess of iodine or acute thyroiditis.20 Hypothyroidism is two to four times more common than hyperthyroidism. (2)
In hypothyroid patients with a strong clinical indication for amiodarone, the drug may be continued with appropriate thyroid hormone supplementation. Treatments of amiodarone-induced hyperthyroidism include the withdrawal of amiodarone (if this can be done safely), the addition of antithyroid medications or prednisone, and surgical thyroidectomy. (20)
LIVER TOXICITY
Liver toxicity, manifested by elevation of liver transaminase levels, is common in patients who are receiving long-term amiodarone therapy. This adverse effect occurs at a rate of 0.6 percent annually. (11)
Patients with liver toxicity are rarely symptomatic. If liver enzyme levels are three times higher than normal, amiodarone should be discontinued unless a patient is at high risk for recurrence of life-threatening arrhythmia. (2)
GASTROINTESTINAL ADVERSE EFFECTS
Gastrointestinal side effects of amiodarone include nausea, anorexia, and constipation. These symptoms often are dosage related and usually improve when the dosage is reduced.
OCULAR ADVERSE EFFECTS
Corneal microdeposits are visible on slit-lamp examination in nearly all patients treated with amiodarone. (19) These deposits seldom affect vision and rarely necessitate discontinuation of the drug.
Optic neuropathy and optic neuritis, sometimes progressing to total blindness, have been described in a small number of patients treated with amiodarone. A causal relationship is not well established. Any patient who notes changes in visual acuity or peripheral vision should be referred for ophthalmologic evaluation.
DERMATOLOGIC ADVERSE EFFECTS
Photosensitivity is common in patients receiving amiodarone therapy. Therefore, all patients should be cautioned to use sunblock and, whenever possible, to cover exposed skin when they are outdoors.
In patients with extended and recurrent sun exposure, bluish skin discoloration may develop in exposed areas. The discoloration resolves over several months after amiodarone is discontinued.
NEUROLOGIC TOXICITY
Neurologic toxicity associated with amiodarone therapy can include ataxia, paresthesias, and tremor. These conditions often are dosage related and improve when the dosage is reduced. Peripheral neuropathy has been reported to occur at a rate of 0.3 percent annually. (11)
CARDIOVASCULAR ADVERSE EFFECTS
Bradycardia and heart block occur in 1 to 3 percent of patients receiving amiodarone. (2) Amiodarone-induced proarrhythmia occurs at an annual rate of less than 1 percent. (11) Although almost all patients treated with the drug have prolongation of the QT interval, polymorphic ventricular tachycardia (i.e., torsades de pointes) is rare. Amiodarone therapy is contraindicated in patients with second- or third-degree heart block who do not have a pacemaker.
Intravenously administered amiodarone causes heart block or bradycardia in 4.9 percent of patients and hypotension in 16 percent. (2) If these conditions occur, infusion of the drug should be discontinued, or the rate of infusion should be reduced.
Intravenous amiodarone therapy should not be used in patients with bradycardia or heart block who do not have a pacemaker. Because phlebitis may occur, the drug should be given through a central venous line when possible.
Drug Interactions
Amiodarone is a potent inhibitor of the hepatic and renal metabolism of several drugs (Table 3). (4,21-25) Amiodarone inhibits metabolism through several cytochrome P450 pathways, including CYP 2C9 (which metabolizes warfarin [Coumadin]), CYP 2D6 (which metabolizes several beta blockers and narcotics), and CYP 3A4 (which metabolizes cyclosporine [Sandimmune] and calcium channel blockers). Interactions with warfarin and digoxin are the most clinically important.
Amiodarone reduces warfarin clearance and can lead to sudden and pronounced increases in the prothrombin time and International Normalized Ratio.21 The peak effects of interaction occur approximately seven weeks after initiation of therapy.
Digoxin levels predictably double after coadministration with amiodarone. (22) This increase occurs because of the inhibition of digoxin secretion from renal tubules and the inhibition of the P-glycoprotein membrane transporter system. (23) The digoxin dosage should be reduced by 50 percent when amiodarone is started, and plasma digoxin levels should be monitored closely.
Patients taking amiodarone should not eat grapefruit or drink grapefruit juice because it can inhibit the conversion of amiodarone to an active metabolite.
Dosage and Administration
In patients receiving oral amiodarone therapy, there may be a delay of two weeks or more before antiarrhythmic effects are noted. A loading regimen (i.e., use of a relatively high dosage at the beginning of therapy) can shorten the delay.
Typical dosing regimens are provided in Table 1.10 Because dosages below 300 mg per day are associated with a reduced incidence of pulmonary adverse effects, physicians should aim for a long-term maintenance dosage of 200 mg per day or less. (18)
Monitoring
Patients treated with amiodarone should be followed regularly to assess ongoing need for amiodarone, efficacy of the drug, appropriateness of dosage, adverse effects, and potential drug interactions. Consensus follow-up recommendations from the NASPE are summarized in Table 4.4 A form to guide patient monitoring is provided in Figure 1.
[FIGURE 1 OMITTED]
The author indicates that he does not have any conflicts of interest. Sources of funding: none reported.
Table 4
Follow-up of Patients Treated with Amiodarone (Cordarone)
Baseline assessment
Complete history and physical examination, with special
attention to congestive heart failure, arrhythmia symptoms, and
concomitant medications
Chest radiograph
Thyroid studies and liver transaminase levels
Digoxin level, prothrombin time, and INR, when appropriate
Pulmonary function tests, including Dlco
Ophthalmologic examination (if preexisting visual impairment)
During outpatient loading
Close surveillance of heart rate, especially during first week
of treatment
History and physical examination directed at detecting
anticipated adverse effects
Every six months
Thyroid studies and liver transaminase levels
Digoxin level as appropriate
History and physical examination directed at detecting anticipated
adverse effects
Suspected pulmonary toxicity
Chest radiograph
Pulmonary function tests
Visual symptoms
Ophthalmologic examination
During warfarin (Coumadin) therapy
Close monitoring of prothrombin time and INR (at least once a week
during first six weeks of treatment)
Suspected digoxin toxicity
Digoxin level
INR = International Normalized Ratio; Dlco = diffusing capacity of
lung for carbon monoxide.
Information from reference 4.
REFERENCES
(1.) Pourbaix S, Berger Y, Desager JP, Pacco M, Harvengt C. Absolute bioavailability of amiodarone in normal subjects. Clin Pharmacol Ther 1985;37: 118-23.
(2.) Physicians' desk reference. 56th ed. Montvale, N.J.: Medical Economics, 2002.
(3.) Libersa CC, Brique SA, Motte KB, Caron JF, Guedon-Moreau LM, Humbert L, et al. Dramatic inhibition of amiodarone metabolism induced by grapefruit juice. Br J Clin Pharmacol 2000;49:373-8.
(4.) Goldschlager N, Epstein AE, Naccarelli G, Olshansky B, Singh B. Practical guidelines for clinicians who treat patients with amiodarone. Practice Guidelines Subcommittee, North American Society of Pacing and Electrophysiology. Arch Intern Med 2000;160:1741-8.
(5.) Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Amiodarone Trials Meta-Analysis Investigators. Lancet 1997; 350:1417-24.
(6.) Lee DS, Green LD, Liu PP, Dorian P, Newman DM, Grant FC, et al. Effectiveness of implantable defibrillators for preventing arrhythmic events and death: a meta-analysis. J Am Coll Cardiol 2003;41: 1573-82.
(7.) Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877-83.
(8.) Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators [Published erratum appears in N Engl J Med 2000;342:1300]. N Engl J Med 1999;341:1882-90.
(9.) Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med 1996;335:1933-40.
(10.) Fuster V, Ryden LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. Circulation 2001;104:2118-50.
(11.) Connolly SJ. Evidence-based analysis of amiodarone efficacy and safety. Circulation 1999;100: 2025-34.
(12.) Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med 2000; 342:913-20.
(13.) Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825-33.
(14.) Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347:1834-40.
(15.) Gallik DM, Singer I, Meissner MD, Molnar J, Somberg JC. Hemodynamic and surface electrocardiographic effects of a new aqueous formulation of intravenous amiodarone. Am J Cardiol 2002; 90:964-8.
(16.) Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Part 6: advanced cardiovascular life support: section 1: introduction to ACLS 2000: overview of recommended changes in ACLS from the guidelines 2000 conference. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation 2000;102 (8 suppl):I86-9.
(17.) Dorian P, Cass D, Schwartz B, Cooper R, Gelaznikas R, Barr A. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation [Published erratum appears in N Engl J Med 2002;347:955]. N Engl J Med 2002;346:884-90.
(18.) Hilleman DE, Spinler SA. Conversion of recent-onset atrial fibrillation with intravenous amiodarone: a meta-analysis of randomized controlled trials. Pharmacotherapy 2002;22:66-74.
(19.) Pollak PT. Clinical organ toxicity of antiarrhythmic compounds: ocular and pulmonary manifestations. Am J Cardiol 1999;84:37R-45R.
(20.) Klein I, Ojamaa F. Thyroid hormone and the cardiovascular system. N Engl J Med 2001;344:501-9.
(21.) Sanoski CA, Bauman JL. Clinical observations with the amiodarone/warfarin interaction: dosing relationships with long-term therapy. Chest 2002; 121:19-23.
(22.) Freitag D, Bebee R, Sunderland B. Digoxin-quinidine and digoxin-amiodarone interactions: frequency of occurrence and monitoring in Australian repatriation hospitals. J Clin Pharm Ther 1995;20: 179-83.
(23.) Yamreudeewong W, DeBisschop M, Martin L, Lower D. Potentially significant drug interactions of class III antiarrhythmic drugs. Drug Saf 2003;26: 421-38.
(24.) Zocor [package insert]. West Pont, Pa.: Merck & Co., Inc., 2003.
(25.) Cheitlin MD, Hutter AM Jr, Brindis RG, Ganz P, Kaul S, Russell RO Jr, et al. ACC/AHA expert consensus document. Use of sildenafil (Viagra) in patients with cardiovascular disease. American College of Cardiology/American Heart Association [Published erratum appears in J Am Coll Cardiol 1999; 34:1850]. J Am Coll Cardiol 1999;33:273-82.
Richard W. Sloan, M.D., R.Ph., coordinator of this series, is chairman and residency program director of the Department of Family Medicine at York (Pa.) Hospital and clinical associate professor in family and community medicine at the Milton S. Hershey Medical Center, Pennsylvania State University, Hershey, Pa.
LYLE A. SIDDOWAY, M.D., is director of the cardiac electrophysiology laboratory at York (Pa.) Hospital and assistant clinical professor in the Department of Medicine at Pennsylvania State University College of Medicine, Hershey. After receiving his medical degree from Johns Hopkins University School of Medicine, Baltimore, Dr. Siddoway completed an internal medicine residency and clinical pharmacology and cardiology fellowships at Vanderbilt University Medical Center, Nashville.
Address correspondence to Lyle A. Siddoway, M.D., 25 Monument Rd., Suite 200, York, PA 17403 (e-mail: lsiddoway@post.harvard.edu). Reprints are not available from the author.
COPYRIGHT 2003 American Academy of Family Physicians
COPYRIGHT 2003 Gale Group