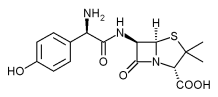
* OBJECTIVE To compare the efficacy of amoxicillin vs placebo in patients with an acute upper respiratory tract infection and purulent rhinorrhea.
* STUDY DESIGN Double-blind randomized placebo-controlled trial.
* POPULATION The 416 patients included from 69 family practices were 12 years or older, presenting with acute upper respiratory complaints, and having a history of purulent rhinorrhea and no signs of complications of sinusitis.
* OUTCOMES MEASURED Therapy success (disappearance of symptoms that most greatly affected the patient's health) at day 10 and duration of general illness, pain, and purulent rhinorrhea.
* RESULTS Therapy was successful in 35% of patients with amoxicillin and in 29% of patients with placebo (relative risk [RR] 1.14, 95% confidence interval [CI], 0.92-1.42). There was no effect on duration of general illness or pain. Duration of purulent rhinorrhea was shortened by amoxicillin (9 days vs 14 for clearing of purulent rhinorrhea in 75% of patients; P = .007). Diarrhea was more frequent with amoxicillin (29% vs 19%, RR 1.28, 95% CI, 1.05-1.57). No complications were reported. One patient (0.5%) receiving amoxicillin and 7 (3.4%) receiving placebo discontinued trial therapy because of exacerbation of symptoms (RR 0.25, 95% CI 0.04-1.56, P = .07). All 8 patients recovered with antibiotic therapy.
* CONCLUSIONS Amoxicillin has a beneficial effect on purulent rhinorrhea caused by an acute infection of the nose or sinuses but not on general recovery. The practical implication is that all such patients, whatever the suspected diagnosis, can be safely treated with symptomatic therapy and instructed to return if symptoms worsen.
* KEY WORDS Respiratory tract infections; sinusitis; antibiotics; therapeutics; family practice. (J Fam Pract 2002; 51:317-323)
**********
KEY POINTS FOR CLINICIANS
* In patients with an acute upper respiratory tract infection that includes purulent rhinorrhea, treatment with amoxicillin has no effect on general recovery and increases the frequency of diarrhea.
* In most patients, symptoms of acute respiratory tract infection last for more than 10 days.
* Treatment without antibiotics and with appropriate follow-up is safe.
* Patients with purulent rhinorrhea caused by an acute infection of the nose or sinuses can initially be treated with symptomatic therapy, whatever the suspected diagnosis, and instructed to return if symptoms worsen.
Infections of the nasal passages are very common (1) and among the most frequent reasons for the prescription of antibiotics. (2,3) Such infections comprise diagnoses that include upper respiratory tract infection (URTI), rhinitis, rhinopharyngitis, and rhinosinusitis, which are very difficult to distinguish because of the lack of specific clinical features or simple office-based diagnostic tests. (4-7) These diagnostic difficulties probably explain why it remains unclear whether and when antibiotics should be used for such patients in clinical practice.
Although evidence shows that a small minority of patients benefit from antibiotic therapy, these patients are extremely difficult to recognize or identify. Three meta-analyses (8-10) on the effect of antibiotics in rhinosinusitis and 5 of 6 recent trials investigating the effect of antibiotics in rhinosinusitis, (11-13) rhinitis, (14) and bacterial rhinopharyngitis (15) almost exclusively studied patients with a diagnosis established by laboratory or imaging investigation. As a result, implementing the findings is difficult in daily practice, where radiologic or laboratory tests are not obtained for most patients with respiratory infections. Only 1 of the 6 trials (16) included patients with a set of clinical symptoms indicating rhinosinusitis. Because inclusion criteria were rather stringent, however, findings are applicable only to a small group of patients.
The purpose of this trial was to investigate the benefits of antibiotic therapy in a larger group of patients with nose or sinus infectious, thereby making the results more widely applicable. Accordingly, we conducted a randomized, double-blind, placebo-controlled trial comparing the effect of amoxicillin with that of placebo in family practice patients with an acute upper respiratory tract infection and presenting with purulent rhinorrhea. Purulent rhinorrhea was chosen as the minimal criterion because it is the symptom moat consistently associated with rhinosinusitis in diagnostic studies. (5,17-21) and because its presence often leads family physicians (FPs) to prescribe antibiotics. (23-26) The trial was designed as a pragmatic effectiveness trial. Patient inclusion and evaluation were defined on a purely clinical basis to maximize relevance for routine daily practice.
METHODS
Study Population
Between October 1996 and December 1999, 69 FPs in Flanders, Belgium, agreed to enroll patients meeting the following inclusion criteria: age 12 years or older, presenting with a respiratory tract infection, and having purulent rhinorrhea. Exclusion criteria were allergy to penicillin or ampicillin; having received antibiotic therapy within the previous week; complaints lasting for more than 30 days; abnormality on clinical chest examination; complications of sinusitis (facial edema or cellulitis; orbital, visual, meningeal, or cerebral signs) (27); pregnancy or lactation; comorbidity that might impair immune competence; and inability to follow the protocol because of language or mental problems. The Ethics Committee of the Ghent University Hospital (GUH) approved the study. All patients (or their guardians, for those younger than 16 years of age) gave written informed consent.
Treatment Assignment and Masking
In this double-blind trial, patients were assigned via a computer-generated random number list to receive 500 mg amoxicillin 3 times a day or placebo for 10 days. The trial medication was supplied in numbered uniform cardboard boxes, each containing 30 capsules of the same size, color, and shape for active and placebo treatment. The randomization list, kept at the pharmacy of GUH, was accessible to the participating FPs only in case of a serious adverse event.
To assess the effectiveness of masking, patients and their FPs guessed the treatment group at 10-day follow-up. Data were encoded and entered without knowledge of treatment allocation. Compliance was assessed by counting leftover medication. All patients were allowed to use xylometazoline 1% nose drops and paracetamol or ibuprofen to alleviate symptoms; these data were registered.
Assessment of Potential Recruitment Bias Caused by Exclusion
First, we compared the characteristics of patients enrolled by high-recruiting FPs (at least 14 patients recruited) with those of patients from low recruiters (at most 5 patients recruited). Second, we asked all participating FPs to complete a short questionnaire over a 6-week period on all patients eligible for the trial but not included in it (sex, age, body temperature, severity of nasal discharge and pain, reason for non-recruitment). Third, to estimate the proportion of sinusitis cases among included patients, all patients were invited for an optional radiologic examination of the maxillary sinuses (single Waters view). (28) Radiographs were taken in the nearest radiology unit, collected centrally, and evaluated by a radiologist of the GUH who specialized in the ear, nose, and throat.
Baseline Measurements
Randomized patients completed an extensive questionnaire and were physically examined by their FP. To evaluate the symptoms, we used the 20 items of the sinonasal outcome test (SNOT-20) (29,30) supplemented by 3 questions about pain. Symptoms were scored on a 6-category (0-5) Likert scale. Patients were also asked to indicate which of their symptoms (no more than 5) were most troublesome.
Follow-Up
During 10 days of treatment, all patients recorded their daily drug intake (trial medication and symptomatic medication); their general feeling of illness; the presence of nasal discharge, pain, and cough; body temperature; the occurrence of presumed adverse drug effects; and absence from work or school. On day 10 they underwent a second physical examination and completed the symptom questionnaire again. In case of insufficient recovery, the FP was then at liberty to prescribe an open antibiotic course (we recommended amoxicillin clavulanate) without revealing the previous treatment phase. Patients who had recovered on day 10 did not have to return on day 15. Any patient with poor recovery on day 10 was asked, regardless of open antibiotic treatment, to continue writing in the diary and to come back on day 15 if complaints were still present.
The 2 primary endpoints were the therapy success rate on day 10 and the duration of general illness, pain, and purulent rhinorrhea as recorded in the diary. Treatment was considered successful when all symptoms that the patient had included in the list of "most important item affecting my health" scored 0 (absent) or 1 (very mildly present) after 10 days of treatment. Secondary endpoints were the mean change in severity score between day 1 and 10 on the various symptoms, incidence of unfavorable evolution, incidence of side effects, intake of analgesics, and duration of sick leave. The number of patients needed to demonstrate a difference in the therapy success rate of 15% at day 10 ([alpha] = 0.05, [beta] = 0.20) was 168 per treatment group. (31) This determination assumed a success rate of 50% in the placebo group. (11,12)
Statistics
Data were analyzed with SPSS-7. Differences in proportions are presented as relative risks with 95% confidence intervals and tested by chi-square test. The duration of symptoms is presented by Kaplan-Meier survival plots. Differences in duration are tested by the log rank test. Other continuous variables are tested by Student's t test or the nonparametric Mann-Whitney U test.
RESULTS
Participant Flow and Follow-Up
Of 416 patients enrolled in the study, 8 were excluded after randomization. Of the 408 patients remaining, 202 received amoxicillin and 206 placebo; 34 patients (8%) withdrew from the trial. Their personal characteristics and clinical conditions at inclusion were not different from those of patients with follow-up. Figure 1 lists reasons for exclusion or withdrawal. The treatment code was broken once for a suspected allergic reaction and once because of an exacerbation of symptoms. In accordance with the intention-to-treat principle, all enrolled patients were included in the analyses in the groups to which they were originally randomized. Patients who had withdrawn because of side effects were also included in the analysis of side effects.
[FIGURE 1 OMITTED]
Complete or partial follow-up data were obtained for 374 patients (90%) after 10 days (mean 10.3 days, standard deviation 1.44): 334 patients completed the questionnaire, 348 returned the diary, and 338 underwent a physical examination. In 265 (71%) patients, data (questionnaire, diary, and physical examination) were complete; in 109 (29%), data at day 10 were partly missing. The two treatment groups were very similar in terms of sex, age, duration of preinclusion complaints, and frequency of various physical signs and symptoms (Table 1). *
Primary Outcomes
Of the 374 patients with follow-up data on day 10, 334 completed the symptom questionnaire twice. Treatment was successful--deFined as a score of 0 (absent) or 1 (very mildly present) for all symptoms that had been included as "the most important item affecting my health"--in 35% of patients in the amoxicillin group (59/170) and 29% in the placebo group (47/164) (Table 2). Relative risk of success was 1.14 (95% CI, 0.92-1.42, P = .24): more patients were cured in the amoxicillin group, but this difference was not statistically significant.
In 82 (19.7%) of the 416 randomized patients (37 amoxicillin, 45 placebo), data on this main outcome are missing. In 40 of these 82 patients, follow-up data are available from the diary (n = 38) or physical examination (n = 2). According to these data, in 13/17 of the amoxicillin group and 11/23 of the placebo group the outcome was favorable: in the diary, the patient reports feeling "well" again at day 10 or sooner, or on physical examination, all signs of respiratory infection have cleared). Eight patients withdrew for clinical exacerbation and 2 patients after full recovery. Adding the 50 patients with a known course of illness to those in the treatment and result groups does not alter the overall result (RR 1.20, 95% CI, 0.98-1.47, P = .08). Furthermore, when considering the 24 nonexcluded patients (13 amoxicillin, 11 placebo) with total lack of follow-up in their allocated treatment group, lust as treatment failures (RR 1.18, 95% CI, 0.97-1.44, P = .11) and then as successes (1.20, 95% CI, 0.99-1.46, P = .07), the result also remains the same. Regarding the success rate from the complete diary data (n = 348) and the results of physical examinations (n = 338) (Table 3), we find no significant difference between treatment groups.
Duration of purulent rhinorrhea was significantly shorter in the amoxicillin group than in the placebo group (75% of patients were free of purulent rhinorrhea after 9 days versus after 14 days in the placebo group, log rank P = .007). There is no difference between treatment groups in the duration of general illness or pain (Figure 2).
[FIGURE 2 OMITTED]
Secondary Outcomes
The mean score reduction on the symptom "thick nasal discharge" between day 1 and day 10 is significantly larger in the amoxicillin group than in the placebo group (2.2 vs 1.5, Student's t test: P <.0001) (Table 3). There is no significant difference in change for any other symptom. Seven patients in the placebo group (3.4%) withdrew before day 10 because of exacerbation of symptoms versus 1 patient (0.5%) in the amoxicillin group (RR 0.25, 95% CI, 0.04-1.56, P = .07). All 8 patients recovered after starting open antibiotic therapy and had no complications or referrals.
The chance of receiving open antibiotic treatment at day 10 follow-up (n = 34: 19 placebo, 15 amoxicillin) or of having to return because of persistent complaints at day 15 (n = 73: 41 placebo, 32 amoxicillin) was not significantly different between the treatment groups (chi-squared test: P = .46 and P = .26, respectively). Diarrhea was more frequent in the amoxicillin group (29% vs. 19%, RR 1.28, CI 1.05-1.57, P = .02). There was no difference in incidence of skin rash, abdominal pain, or vomiting. Absence from work or school was comparable in both treatment groups (RR 0.95, 95% CI, 0.86-1.05, P = .34). Patients in the amoxicillin group took an analgesic an average of 5 times, mainly in the first days of treatment, compared with 4 for the placebo group (Mann-Whitney U test,, P = .24).
Other Results
The lack of correlation between the estimated and actual treatment demonstrates that masking was maintained. Compliance was good in both groups: 89% of patients in the amoxicillin group and 91% of those in the placebo group took at least 25 of 30 capsules.
Patients from low recruiters were not significantly different from patients enrolled by high recruiters. Included patients had slightly more complaints of pain (58% vs 50%, RR 1.20, CI 1.02-1.42, P = .03) than the 332 eligible but excluded patients registered during the 6-week period. The most frequent reasons for exclusion were the presence of an exclusion criterion (22%), the patient's refusal to participate (16%), the patient's request for antibiotic therapy (14%), and lack of time by the FP (10%). Of the 292 patients who agreed to undergo a radiologic examination, about two thirds had abnormalities of the maxillary sinuses.
DISCUSSION
This study produced 3 important findings. First, we found that patients consulting their FP for acute URTI with purulent rhinorrhea do not experience any important benefit from amoxicillin therapy. With treatment, the purulent rhinorrhea disappears more quickly, but this seems to be of little importance in relation to a general recovery. Moreover, amoxicillin therapy increases the risk of diarrhea. We further found that with or without amoxicillin, complaints last long: after 10 days, two thirds of patients still had complaints and about half of the patients still felt ill. The natural course to recovery takes a long time and is not influenced by taking amoxicillin. Finally, we observed that failure to prescribe antibiotics is safe. The placebo group had no complications. A small number of exacerbations occurred, but these responded swiftly to a course of amoxicillin-clavulanate.
To our knowledge, this is the fast time that the effect of an antibiotic in adult patients presenting with acute purulent rhinorrhea (but with an otherwise unspecified diagnosis) has been investigated in a randomized, placebo-controlled trial. This trial is in line with a number of other family practice-based pragmatic trials in which patients were included on the basis of respiratory symptoms instead of by diagnosis (16,32-37) and in which the emphasis was on practical relevance rather than on diagnostic accuracy.
Since 1995, 6 randomized clinical trials of high methodologic quality (11-16) have studied the efficacy of antibiotics in general practice patients suffering from various acute infections of the nasal passages and usually presenting with purulent rhinorrhea. In 3 of these trials, no beneficial effect of antibiotics was found. Study populations consisted, respectively, of patients with a set of clinical symptoms (including purulent rhinorrhea) indicating rhinosinusitis (16); patients with clinical suspicion of rhinosinusitis plus sinus abnormalities on conventional radiology (11); and patients with clinical suspicion of sinusitis but without the radiologic signs. (14) In the 3 other trials, treatment was (more or less) effective. Included were patients with clinical suspicion of sinusitis and abnormalities on CT scan, (12) patients with unilateral facial pain and elevated C-reactive protein levels or erythrocyte sedimentation rate, (13) and patients with rhinopharyngitis and positive bacteriologic cultures of nasopharyngeal secretions. (15) These trials show that antibiotics are efficacious in some patients. In our trial, which probably included a mix of all these populations, we also found more patients in the amoxicillin group to be symptom free after 10 days. Despite a fairly large sample size, however, this difference was too small (less than 15%) to be statistically significant.
In this trial, as in daily practice, we did not know the precise diagnosis of included patients. Moreover, despite our frequent requests, participating FPs included only a minority of eligible patients. Concern might arise that only patients with mild disease were studied. We made 3 efforts to verify that the population was truly representative. First, we determined that the personal characteristics and severity of symptoms of patients of low-recruiting FPs (who tend to include patients with worse symptoms (38)) were no different from those of patients included by high recruiters. Second, an analysis of questionnaires from all eligible but excluded patients over a 6-week period showed that included and excluded patients were very much alike. The analysis also showed that in only 3% of patients did the FP consider the subject too ill to be included. Third, the results obtained on plain radiography of the maxillary sinuses were in line with the imaging results of other family practice populations with clinical suspicion of rhinosinusitis. (11,19-21)
With regard to the methodology, we wish to clarify certain choices. Amoxicillin was selected because it ks recommended as the first-line drug for rhinosinusitis in several practice guidelines (39-41) and the ,sensitivity of respiratory pathogens to it was sufficient in our geographic area at the start of the trial. (42) * To evaluate symptoms, we chose the 20 items of the SNOT-20 questionnaire (Table 1), an abbreviated version of the RSOM-31, (29) a disease-specific quality-of-life test for sinusitis. These 20 items include not only all classic rhinosinusitis symptom,; but also a number of more subjective symptoms, such as sleep disturbances and reduced productivity, which may also severely inconvenience patients. Any beneficial effect of amoxicillin on these symptoms would be just as important as an effect on the classic sinusitis symptom.
Outcome measures were mainly self-assessed by patients, since in this kind of pathology, for which subjective inconvenience is often greater than objective signs might indicate, the patient is in our view the best and only judge of symptom improvement. The main outcome measure, disappearance of perceived worst symptoms, was designed to take into account the heterogeneity of clinical presentations.
CONCLUSIONS
Patients with an acute upper respiratory tract infection with purulent rhinorrhea (and without signs of complications of sinusitis) represent a large, clearly defined, clinically recognizable group. Our results show that amoxicillin provides no clinically important benefits for this population. The implication for practice is that whatever diagnosis is suspected, all these patients can safely be treated with symptomatic therapy only. Patients should, however, be informed that whichever treatment is chosen, symptoms can last for a long time. In the rare event that symptoms worsen, they should consult their FP for antibiotic therapy. If patients are dearly distressed by the purulent rhinorrhea itself, this trial suggests reasons for considering the use of amoxicillin, but potential patient benefits still probably do not outweigh the disadvantages.
ACKNOWLEDGMENTS
The authors wish to thank all participating family physicians and patients and Erna Eeckhout, Adrienne Dubron, Anselme Derese, MD, Ph D, and John Marshall for their invaluable help.
* For an expanded version of this table, see Table W1 at http://www.jfponline.com.
* Streptococcus pneumoniae 97% sensitive and Haemophilus influenzae 87% sensitive: data from Ghent University Hospital, Laboratory of Bacteriology, De Pintelaan 185, B-9000 Ghent, Belgium. Director: Prof G. Verschraege Personal communication.
REFERENCES
(1.) Okkes IM, Oskam SK, Lamberts H. Van klacht naar diagnose. Episodegegevens uit de huisartspraktijk. Coutinho, Bussum, Nl; 1998.
(2.) De Maeseneer J. Her voorschrijven van antibiotica bij luchtwegproblemen. Een explorerend onderzoek. Huisarts Wet 1990; 33:223-6.
(3.) De Melker R,A, Kuyvenhove MM. Management of upper respiratory tract infection in Dutch general practice. Br J Gen Pract 1991; 41:504-7.
(4.) van Buchem L, Peeters M, Beaumont J, Knottnerus JA. Acute maxillary sinusitis in general practice: the relation between clinical picture and objective findings. Eur J Gen Pract 1995; 1:155-60.
(5.) Hansen JC, Schmidt H, Rosborg J, Lund E. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995; 311:233-6.
(6.) Stalman W, Van Essen GA, Gubbels JW, De Melker RA. Difficulties in diagnosing acute sinusitis in a Dutch group practice. Relative value of history, radiography and ultrasound. Eur J Gen Pract 1997; 3:12-5.
(7.) Hueston WJ, Mainous AG, Dacus EN, Hopper JE. Does acute bronchitis really exist? A reconceptualization of acute viral respiratory infections. J Fam Pract 2000; 49:401-6.
(8.) Ferranti de SD, Ioannidis JPA, Lau J, Anninger WV, Barza M, Are amoxicillin and folate inhibitors as effective as other antibiotics for acute sinusitis? A meta-analysis. BMJ 1998; 317:632-7.
(9.) Williams JW Jr, Aguilar C, Makela M, et al. Antibiotic therapy for acute sinusitis: a systematic literature review. In: Douglas R, Bridges-Webb C, Glasziou P, Lozano J, Steinhoff M. Wang E, eds. Acute respiratory infections module of the Cochrane Database of Systematic Reviews. The Cochrane Library. Oxford, England: Updated Software; 1997,
(10.) Zucker DR, Balk E, Engels E, Barza M, Lau J. Agency for Health Care Policy and Research Publication No. 99-E016. Evidence report/technology assessment number 9. Diagnosis and treatment of acute bacterial rhinosinusitis. Available at www.ahrq.gov/clinic/sinussum.htm
(11.) van Buchem FL, Knottnerus JA, Scrhrijnemaekers VJJ, Peeters MF. Primary-care-based randomised placebo-controlled trial of antibiotic treatment in acute maxillary sinusitis. Lancet 1997; 349:683-7.
(12.) Lindbaek M, Hjortdahl P, Johnsen UL-H. Randomised, double blind, placebo controlled trial of penicillin V and amoxycillin in treatment of acute sinus infections in adults. BMJ 1996; 313:325-9.
(13.) Hansen JG, Schmidt H, Grinsted F. Randomised, double blind, placebo controlled trial of penicillin V in the treatment of acute maxillary sinusitis in adults in general practice. Scand J Prim Health Care 2000; 18:44-7.
(14.) Haye R, Lingaas E, Hoivik HO, Odegard T. Azithromycin versus placebo in acute infectious rhinitis with clinical symptoms but without radiological signs of maxillary sinusitis. Eur J Clin Microbiol Infect Dis 1998; 17:309-12.
(15.) Kaiser L, Lew D, Hirschel B, et al. Effects of antibiotic treatment in the subset of common-cold patients who have bacteria in nasopharyngeal secretions. Lancet 1996; 347:1507-10.
(16.) Stalman W, van Essen GA. van der Graaf Y, de Melker RA. The end of antibiotic treatment in adults with acute sinusitis-like complaints in general practice? A placebo-controlled double-blind randomized doxycycline trial. Br J Gen Pract 1997; 47:794-9.
(17.) Axelsson A, Runze U. Symptoms and signs af acute maxillary sinusitis. ORL J Otorhinolaryngol Relat Spec 1976; 38:298-308.
(18.) Berg O. Carenfelt C. Analysis of symptoms and clinical signs in the maxillary sinus empyema. Acta Otolaryngol (Stockholm) 1988; 105:343-9.
(19.) Lindbaek M, Hjortdahl HR, Johnsen UL-H. Use of symptoms, signs and bloodtests to diagnose acute sinus infections in primary care: comparison with computed tomography. Fam Med 1996; 28:181-6.
(20.) Van Duyn NP, Brouwer HJ, Lamberts H. Use of symptoms and signs to diagnose maxillary sinusitis in general practice: comparison with ultrasonography. BMJ 1992; 305:684-7.
(21.) Williams JW, Simel DL, Leroy R, Samsa GP. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Int Med 1992; 117:705-10.
(22.) Axelsson A, Runze U. Comparison of subjective and radiological findings during the course of acute maxillary sinusitis. Ann Otol Rhinol Laryngol 1983; 92:75-7.
(23.) Gonzales R, Barrett PH, Steiner JF. The relation between purulent manifestations and antibiotic treatment of upper respiratory tract infections. J Gen Intern Med 1999; 14:151-6.
(24.) Little DR, Mann BL, Sherk DW. Factors influencing the clinical diagnosis of sinusitis. J Fam Pract 1998; 46:147-52.
(25.) Mainous AG, Hueston WJ, Eberlein C. Colour of respiratory discharge and antibiotic use. Lancet 1997; 350:1077.
(26.) De Sutter AI, De Meyere MJ, De Maeseneer JM, Peersman WP. Antibiotic prescribing in acute infections of the nose or sinuses: a matter of personal habit? Fam Pract 2001; 18:209-13.
(27.) Gray WC, Blanchard CL. Sinusitis and its complications. Am Fam Physician 1987; 35:232-43.
(28.) Williams JW, Roberts L, Distell B, Simel DL. Diagnosing sinusitis by X-ray: is a single Waters view adequate? J Gen Intern Med 1992; 7:481-5.
(29.) Piccirillo JF, Edwards D, Haiduk A. et al. Psychometric and clinimetric validity of the 31-item rhinosinusitis outcome measure (RSOM-31). Am J Rhinol 1995; 9:297-306.
(30.) Bhattacharyya T, Piccirillo J, Wippold FJ II. Relationships between patient-based descriptions of sinusitis and paranasal sinus computed tomographic findings. Arch Otolaryngol Head Neck Surg 1997; 123:1189-92.
(31.) Altman DG. Practical statistics for medical research. London, England: Chapman & Hall; 1991: 455-60.
(32.) De Meyere M. Acute keelpijn in de eerste lijn. [dissertatie]. 1990 Rijksuniversiteit Gent, Faculteit Geneeskunde.
(33.) Verheij TJM, Hermans J, Mulder JD. Effects of doxycycline in patients with acute cough and purulent sputum: a double blind placebo controlled trial. Br J Gen Pract 1994; 44:400-4.
(34.) Burke P. Bain J, Robinson D, Dunleavy J. Acute red ear in children: controlled trial of non-antibiotic treatment in general practice. BMJ 1991; 303:558-62.
(35.) Damoiseaux RAMJ, Balen van FAM, Hoes AW, Melker de RA. Primary care based randomised, double blind trial of amoxicillin versus placebo for acute otitis media in children aged under 2 years. BMJ 2000; 320:350-4.
(36.) Little P, Could C, Williamson I, Moore M, Warner G, Dunleavy J. Pragmatic randomised controlled trial of two prescribing strategies for childhood acute otitis media. BMJ 2001; 322:336-42.
(37.) Little P, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. Open randomised trial of prescribing strategies in managing sore throat. BMJ 1997; 314:722-7.
(38.) Wilson S, Delaney BC, Roalfe A, et al. Randomised controlled trials in primary care: case study. BMJ 2000; 321:24-7.
(39.) De Bock GH, Van Duijn NP, Dagnelie CF, et al. NHG-Standaard Sinusitis. Huisarts Wet 1993;36:255-7.
(40.) Low DE, Desrosiers M, McSherry J, et al. A practical guide for the diagnosis and treatment of acute sinusitis. CMAJ 1997; 156(suppl 6):S1-14.
(41.) Snow V, Mottur-Pilson C, Hickner JM. principle of appropriate antibiotic use for acute sinusitis in adults. Arm Intern Med 2001; 134:495-7.
(42.) Pierard D, De Meyer A, Vanzeebroeck A, Lauwers S. In vitro evaluatie van de gevoeligheid van 205 recente klinische isolaten van streptococcus pneumoniae voor minocycline en andere antibiotica. Tijdschr Geneesk 1996; 52:281-5.
From the Department of General Practice and Primary Health Care (A.I.DeS., M.J.DeM., T.C.C., M.L.v.D., J.M.DeM.) and the Department of Population Studies and Social Sciences Research Methods (W.P.P.). University of Ghent, Belgium. This study was presented at the 28th annual meeting of the North American Primary Care Research Group, Amelia Island, Fla., November 2000, and at the European meeting of the World Organization of National Colleges, Academies, and Academic Associations of General Practitioners/Family Physicians (WONCA), Tampere, Finland, June 2001. Competing interest: This trial was financed by a grant by Eurogenerics NV, Brussels. Reprint requests should be addressed to An De Sutter, MD, Department of General Practice UG.UZG-1K3, De Pintelaan, 185, B 9000 Ghent, Belgium. E-mail: an.desutter@rug.ac.be.
COPYRIGHT 2002 Appleton & Lange
COPYRIGHT 2002 Gale Group