Headlines over the past few months have consumers reeling:
* Sept. 30, 2004 - Arthritis drug Vioxx, a COX-2 inhibitor, was pulled off the market after a clinical trial showed that it "doubles a person's risk of heart attack and stroke."1 Vioxx was approved by the Food and Drug Administration (FDA) for sale in May 1999.
* Oct. 15, 2004 - The FDA issues a public health advisory directing manufacturers of all antidepressant drugs to revise their product labeling to include a "black box" warning to alert health care providers of an increased risk of suicidal thoughts and behavior in children being treated with the drugs. The announcement affects the entire general class of antidepressant medications, including Prozac (fluoxetine), Zoloft (sertraline), Paxil (paroxetine), Luvox (fluvoxamine), Celexa (citalopram), Lexapro (escitalopram), Wellbutrin (bupropion), Effexor (venlafaxine), Serzone (nefazodone), and Remeron (mirtazapine), among others.2
* Dec. 17, 2004 - A letter to doctors, appearing in The New England Journal of Medicine's Dec. 23, 2004 issue, stated that in light of the findings for Vioxx, clinicians should "stop prescribing" Bextra, a COX-2 inhibitor.3 Bextra was approved for sale by the FDA in 2001.
* Dec. 20, 2004 - The FDA issues an alert that Celebrex use "may be associated with an increased risk of adverse cardiovascular (CV) events, including cardiovascular death, acute myocardial infarction and stroke."4 Celebrex, another COX-2 inhibitor, was approved by the FDA for sale in 1998.
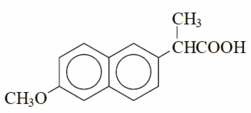
* Dec. 20, 2004 - The FDA warns health care professionals "of the risk of hepatotoxicity" that may be associated with the use of Strattera.5
* Dec. 21, 2004 - The FDA advises patients taking Aleve (Anaprox, Naprelan and Naprosyn) "to take the drugs for no longer than 10 days without consulting a physician." The advisory was issued after the National Institutes of Health (NIH) had halted a study after finding that patients taking Aleve had "50% more heart attacks and strokes."6 Naproxen, the generic name for these drugs, was approved by the FDA for prescription sale in 1976 and for overthe-counter sale in 1994.
Did the FDA wake up, or is it just that there is so much heat on the FDA that important information is finally beginning to be presented to the public?
Consider these two headlines:
* "Scientist Says FDA Called Journal to Block (Negative) Vioxx Article" (USA Today)
* "Some FDA Staff Had Drug Safety Concerns - Survey" (Reuters)
The second headline refers to an internal survey of FDA scientists regarding their opinions of the FDA's effectiveness in ensuring public safety for the drugs it approves. Included in the results of this survey are some startling facts, again coming from the minds of the FDA's own scientists:
* Only 24% of the scientists agree that the six-month time schedule provides enough time to "conduct an in-depth, scientific review" of priority drug submissions.
* A mere 64% are "mostly" or "completely" confident that the FDA's final decisions "adequately assess the safety of a drug."
* An alarming 34% are "mostly" or "completely" confident that the FDA "adequately monitors the safety of prescription drugs once they are on the market."
* A disconcerting 18% of the scientists have been "pressured to approve or recommend approval" for drugs "despite reservations about the safety, efficacy or quality of the drug."
So, what picture does this draw for us? The scientists doing the work don't have enough time to do the job; so much so that some are being pressured to approve drugs they have concerns about. A third doubt the safety of the drugs that are being approved, and two-thirds doubt that the public is protected once the drugs are out there.
How long has this been the situation? Based upon the situation with Aleve, the answer is probably at least three decades. Given all this, what is the responsibility of the chiropractic profession? To give clear warnings and present alternatives.
It is very clear that our patients are making their own health care choices. This is why the drug companies spend so much money on direct-to-consumer advertising. But there are facts that our patients need to know:
1. There is no such thing as a "safe" drug. Every drug has sideeffects, many times including death.
2. Each time they take a pill, they take a risk. The more pills, the more risk. Taking drugs in combination just multiplies the risks exponentially. Sooner or later, those risks are going to result in some kind of adverse reaction.
3. The newer the drug, the less time to reveal dangers. Many of the real dangers are not discovered until the general public has used a drug for some time. Encourage patients to avoid drugs that have been on the market for less than five years, if possible.
4. There are alternatives, lots of them. Consider chiropractic, nutrition, diet, lifestyle changes, etc. Wellness is what the body manifests, not what a drug can invoke.
The chiropractic profession is almost alone in its position as a nondrug advocate. We are the opposing voice that can weigh in and provide an alternative point of view. As individual DCs, this is a day-to-day responsibility. Our patients need to hear about the dangers and alternatives. You are probably the only one they can depend on to provide this valuable advice.
References
1. Arthritis drug Vioxx pulled off market. WebMD Medical News, Sept. 30, 2004.
2. Antidepressants get PDA's "black box" warning. Dynamic Chiropractic, Nov. 30, 2004: www.chiroweb.com/archives/22/25/03.html.
3. Doctors say avoid Pfizer's Bextra - medical journal. Reuters, Dec.
17, 2004.
4. Celebrex trial halted due to adverse cardiovascular events. Medscape Medical News, Dec. 20, 2004.
5. Strattera may cause hepatotoxicity. Medscape Medical News, Dec. 20. 2004.
6. Aleve linked to heart attack, stroke. WebMD Medical News, Dec. 21. 2004.
DMP Jr.
Donald M. Petersen Jr., BC, HCD (hc), FICC (h)
Editor/Publisher of Dynamic Chiropractic(TM)
Don@MPAmedia.com
Copyright Dynamic Chiropractic Feb 12, 2005
Provided by ProQuest Information and Learning Company. All rights Reserved