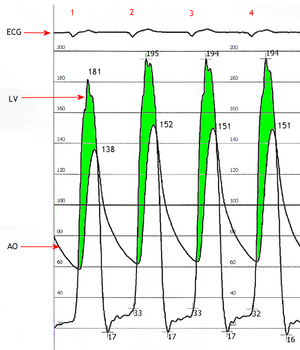
Current medicine has displayed a trend toward less interfering techniques but more invasive surgical approaches in older patients with more comorbidities. In this population, the prevalence of symptomatic cardiac disease including aortic stenosis is increased. More than 25 years have elapsed since severe aortic stenosis was identified as an independent, important risk factor for patients undergoing general anesthesia for noncardiac surgery. Despite impressive advances in anesthesiologic and surgical techniques, morbidity and mortality in patients with severe aortic stenosis remains high. Published study results clearly show that adverse perioperative risk in patients with aortic stenosis depends on the interaction of factors such as the severity of valve disease, concomitant coronary artery disease, and the severity and/or urgency of the surgical procedures. The mainstay of preoperative evaluation remains the obtaining of a comprehensive preoperative medical history and a physical examination, while transthoracic echocardiography is necessary to establish or exclude hemodynamically relevant aortic stenosis in selected patients. Perioperative care is established in patients with asymptomatic aortic stenosis and/or those undergoing low-risk surgery. However, further preoperative testing or aortic valve replacement prior to noncardiac surgery should be discussed individually with the patients awaiting urgent surgical procedures who are at medium or high risk. At this point, decisions should be made in an interdisciplinary manner, including the opinions/wishes of the patient and the patient's family.
Key words: anesthesiology; aortic stenosis; surgery
Abbreviations: CAD = coronary artery disease; LV = left ventricle, ventricular
**********
Current medicine displays a trend toward less interfering techniques but more invasive surgical approaches in older and sicker patients. In this population, the prevalence of symptomatic cardiac disease including aortic stenosis is increased, resulting in an elevated risk for perioperative morbidity and mortality during noncardiac surgery. This overview covers important aspects of preoperative/perioperative care in patients who are at increased risk for or have established aortic stenosis and are facing noncardiac surgery.
PROBLEM DEFINITION: PERIOPERATIVE RISK ASSESSMENT IN NONCARDIAC SURGERY
Cardiac complications due to coronary artery disease (CAD) pose the most important risks to patients during noncardiac surgery: about 25% of patients scheduled for noncardiac surgery have coronary risk factors or known CAD, (1) and up to 12% of high-risk patients undergoing high-risk vascular surgery will have a perioperative myocardial infarction. (2) This contributes to a high morbidity and mortality rate and to a high economic burden due to the required management of those complications. (3)
Since the landmark studies of Goldman et al, (2) and Detsky et al, (4) the physician's ability to identify patients who are at elevated risk for perioperative cardiac complications has increased substantially. Of note, increased perioperative/postoperative risk is dependent on a mixture of both patient-related and procedure-related factors (Table 1).
Although the ultimate goal of presurgical risk evaluation is to reduce the morbidity and mortality associated with surgery, and to restore the patient quickly to the desired level of functioning, it is not prudent to order all available tests for every patient in every situation. This is highlighted by the interesting findings of Schein et al (5): about 19,000 patients who were scheduled for cataract surgery were randomly assigned to be thoroughly evaluated or not to be thoroughly evaluated using several medical tests before surgery. The overall rate of complications was the same in both groups (approximately 3%). Thus, routine medical testing before low-risk procedures does not significantly increase the safety of surgery.
The medical consultant and/or the anesthetist have to rely primarily on the medical history and physical examination, including an evaluation of the patient's functional status, to make preoperative assessments. Subsequently, the consultant must choose from appropriate diagnostic tests (eg, exercise stress testing and stress echocardiography (6)) to confirm his clinical impressions and to account for the risks of the surgical procedures (ie, high-risk vs low-risk procedure or planned vs urgent surgery). (6) Diagnostic tests should only be ordered when preoperative therapeutic strategies such as medical treatment or interventions (eg, percutaneous coronary intervention and coronary artery bypass grafting) appear to be helpful. (6,7)
AORTIC VALVE DISEASE AND CARDIOVASCULAR RISK IN NONCARDIAC SURGERY
Aortic valve stenosis is the most common valvular heart disease in the elderly. (8-10) Degeneration of the aortic cusps evolves with age and the population is aging in industrialized countries. (11) Large studies report that 2 to 9% of adults who are > 65 years of age are affected by aortic stenosis, (8,9) which has major implications for health-care providers. Additionally, approximately 1 to 2% of the general population is born with a bicuspid aortic valve, which is prone to early degeneration and calcification. Thus, even younger patients (ie, those < 65 years old) may be at increased risk for aortic valve stenosis.
The increased perioperative risk during noncardiac surgery is highlighted by the landmark studies of Stewart et al (8) and Lindroos et al, (9) who reported that patients with severe aortic stenosis face a 17.3% risk Of cardiac complications and a 13% mortality rate during noncardiac surgery. Despite impressive advances in anesthesiologic techniques during the past few years, the risks of noncardiac surgery in patients with aortic stenosis remain high (Table 2), (12-15,38,39) as follows:
* In a retrospective cohort study (12) performed between 1991 and 2000, the composite end point of perioperative mortality and nonfatal myocardial infarction was more common in patients with aortic stenosis than in those without it (14% vs 2%, respectively). An adverse outcome was more common in patients with advanced aortic valve disease (patients with an aortic valve area of < 0.7 [cm.sup.2] or a mean gradient of > 50 mm Hg, 31%; patients with an aortic valve area of 0.7 to 1 [cm.sup.2] or a mean gradient of 25 to 49 mm Hg, 11%).
* In a study (13) evaluating the predictive role of transthoracic echocardiography, the relative risk for perioperative cardiac complications was 6.8 in patients with a peak instantaneous gradient of [greater than or equal to] 40 mm Hg.
However, perioperative risks appear to be low in patients undergoing low-risk procedures or in selected patients, as follows:
* Patients with a low risk score for CAD had no adverse perioperative outcomes. (12)
* No deaths occurring during noncardiac surgery were reported in a retrospective analysis (14) of 48 consecutive patients with severe aortic stenosis (mean aortic valve area, 0.6 [cm.sup.2]; 36 symptomatic patients). However, seven patients experienced perioperative events. No patients in a subgroup of 25 patients with severe aortic stenosis who were undergoing noncardiac surgery under local anesthesia experienced a cardiac event.
* Two of 19 elderly patients with severe, symptomatic aortic stenosis had postoperative complications and subsequently died. Hypotensive episodes have been treated promptly in those patients with [alpha]-adrenergic agonists. (15)
Although no systematic, prospective studies are currently available on this topic, these findings clearly show that adverse perioperative risk in patients with aortic stenosis depends (1) the severity of the aortic stenosis, (2) the presence of concomitant CAD, which is present in up to 50% of patients with aortic stenosis, (16-20) and (3) the severity of the surgical procedures, including the involvement of volume shifts and/or the instability of perfusion during systemic hypotension.
PREOPERATIVE RISK ASSESSMENT IN PATIENTS WITH AORTIC STENOSIS
The essential issue during preoperative assessment is to estimate the risk/benefit ratio between the risk of noncardiac surgery and the severity of aortic stenosis. However, different definitions of disease severity have been used in published studies, impeding the simple interpretation of those studies. Irrespective of noncardiac surgery, symptomatic patients with severe aortic stenosis, determined according to the definitions published by an American College of Cardiology/American Heart Association task force, (21) are at a highly increased risk for experiencing adverse events during follow-up. Even asymptomatic patients with severe aortic stenosis display a 1 to 2% risk of sudden cardiac death, while patients with moderate aortic stenosis display an overall risk that is comparable to that of the general "healthy" population. (11,21) On the basis of a variety of data on hemodynamics and natural history, moderate aortic stenosis was defined as an aortic valve area of 1.0 to 1.5 [cm.sup.2] (mean transvalvular gradient, approximately 25 to 50 mm Hg at normal cardiac output), and severe aortic stenosis was defined as an aortic valve area of < 1.0 [cm.sup.2]. When stenosis is severe and cardiac output is normal, the mean transvalvular gradient is generally > 50 mm Hg. (21) The critical reduction of the aortic valve area individually depends on the body height and size of the affected patients, and Doppler echocardiography data should be included for quantification. Asymptomatic patients with a peak transvalvular jet velocity of > 4 m/s have a > 50% likelihood of symptom onset or death within 2 years. (22) None of the latter patients with moderately sized aortic stenoses died suddenly. Adverse events were unlikely to develop during follow-up in patients with a jet velocity of < 3 m/s. (22) All of those data were derived from observational data of large populations, which do not reflect the perioperative situation of patients with aortic stenosis. Nevertheless, we used the definitions cited above for a dear, traceable risk assessment of adult patients, since these definitions appear to give reliable estimates even for valve-related risk during noncardiac surgery, Operative risk stratification should further include the consequences of severe aortic stenosis, such as left ventricular (LV) hypertrophy and LV dysfunction, and the type of noncardiac surgery.
HOW TO DEAL WITH THE HEALTHY PATIENT FACING NONCARDIAC SURGERY
The obtaining of a medical history and a physical examination are the cornerstones of the preoperative evaluation of all patients. It is not feasible and not cost-effective to conduct an echocardiographic study in every patient during the preoperative setting. (14) Cardiac murmurs are common in asymptomatic patients facing noncardiac surgery. Aortic stenosis can be recognized during the physical examination by the presence of a low-frequency, systolic ejection murmur. Other findings may include the following: (1) a soft S2 during auscultation due to a lack of or a delay of aortic valve closure; (2) the arterial pulse described as "parvus and tardus," best appreciated in the carotid artery; and (3) a sustained cardiac impulse at the apex, which is initially normal in the location.
Therefore, the medical consultants, surgeons, and/or anesthesiologists must be trained to distinguish organic from functional systolic murmurs to determine which patients require further quantification of the severity of valvular disease. Due to the large amount of responsibility of the consultant concerning the health and well-being of patients with possible aortic stenosis and the high degree of uncertainty during auscultation, especially in untrained physicians, we recommend conducting echocardiography in every unclear situation, especially in patients with a poor functional status to get exact information about the seriousness of the valve disease (Fig 1). Echocardiographic studies may provide data on whether perioperative antibiotic prophylaxis for endocarditis is required (Tables 3, 4).
[FIGURE 1 OMITTED]
Degenerative aortic valve disease is often associated with CAD. (19-21) Up to 50% of patients with aortic stenosis and typical angina have been reported to display relevant CAD. Even in patients who are < 40 years old and have aortic stenosis with no chest pain and no coronary risk factors, the prevalence of CAD is 3 to 5%. (21) Exercise stress testing or stress echocardiography will probably help to identify affected patients who are at increased risk for CAD (Fig 1). Patients with mild-to-moderate aortic stenosis and a risk for CAD will presumably benefit from perioperative [beta]-blocker treatment, (23) although this has not been systematically evaluated in this situation. In addition, relevant carotid artery disease may be associated with aortic valve stenosis and should be included in the preoperative risk evaluation. If the noncardiac surgery can be postponed and the peak instantaneous gradient is high, we would even perform coronary angiography in order to plan aortic valve replacement with or without coronary bypass grafting before high-risk noncardiac surgery (Fig 1). Noncardiac surgery can be safely performed 3 months after coronary bypass grafting with an overall cardiac risk reduction from 3.3 to 1.7%. (6) There is also a paucity of data addressing the timing of valve repair/replacement prior to elective noncardiac surgery. Conventional practice dictates that the indications are the same as in the absence of noncardiac surgery. (21) Elective patients with severe asymptomatic aortic stenosis usually undergo valve surgery first. Of note, the risks of anticoagulation therapy during neurosurgery and concerns posed by the use of prosthetic valves (ie, infection secondary to abdominal surgery) may represent an indication for a combined procedure in selected eases, with the noncardiac operation performed immediately after the valve surgery. Also, palliative noncardiac surgery (eg, partial cancer resection for relief of symptoms) may be performed in patients with severe aortic stenosis whose short-to-mid-term life expectancy is poor (Fig 1). In selected patients who are at very high risk, a nonsurgical palliative approach may be chosen. Since those individual approaches have not been evaluated systematically, we try to make decisions in an interdisciplinary manner and to include the opinions/ wishes of the patient and the patient's family.
VALUE OF MEDICAL CONSULTATION IN PATIENTS WITH AORTIC STENOSIS
A medical consultant is often contacted at the request of the surgeon or the primary care physician prior to the consideration of a surgical referral. The goals of a medical consultation are (1) to identify unrecognized comorbid disease and (2) to optimize the preoperative medical condition as a member of the preoperative team. This should include recommendations on prophylaxis for venous thrombembolism and endocarditis, especially in patients with aortic stenosis with or without atrial arrhythmias. (24,25)
Obtaining a medical history, performing a physical examination, and ordering specific technical tests for confirmation of the diagnosis, consultants identify medical conditions that are related to surgical outcome, occasionally delaying noncardiac surgery. (26,27) Such procedures may decrease the length of hospital stay during noncardiac surgery.28 However, no study has evaluated whether perioperative morbidity or mortality is improved with perioperative care by medical consultation, and a high degree of misunderstandings between consultants and referring physicians has been reported. (29)
VALUE OF ECHOCARDIOGRAPHY IN ASYMPTOMATIC PATIENTS WITH AORTIC STENOSIS
To our knowledge, the clinical value of echocardiography in asymptomatic patients with aortic stenosis facing noncardiac surgery has not been systematically examined. However, the rates of major adverse events in patients with asymptomatic aortic stenosis have been high in older reports, (2) while the rates of fatal cardiac complications were considerably lower in newer reports, (14,15) even when only patients with severe aortic stenosis have been analyzed. Graduation of aortic stenosis was preoperatively available in the patients of latter studies, but not in the studies of Goldman et al (2) and Detsky et al. (4) Improvements in anesthetic and surgical techniques during the last 20 years and knowledge about the presence and severity of aortic stenosis may be responsible for improved outcomes during noncardiac surgery. One report (15) from the Mayo Clinic in Rochester, MN, confirms our opinion. In this retrospective analysis, the rate of complications was tremendously higher in patients with unrecognized severe aortic stenosis before higher risk surgery compared to those patients who had undergone a preoperative echocardiographic evaluation. The knowledge of the severity of the aortic stenosis appears to be essential for the perioperative and postoperative management of patients. Thus, echocardiographic evaluation should be performed in every patient with suspected aortic stenosis who had been scheduled for surgery.
ECHOCARDIOGRAPHIC MEASURES IN THE DIAGNOSIS OF AORTIC STENOSIS
Echocardiography plays a major role in the evaluation of the severity of aortic stenosis and has replaced cardiac catheterization for the assessment of aortic stenosis in many centers. Catheterization is now performed almost only when coronary angiography is required. (30) The most common cause of aortic stenosis in older patients is degeneration and calcification, while stenosis of the bicuspid valve is more common in younger adults. Two-dimensional echocardiography is helpful in assessing the morphology of the aortic valve and helps in the decision to provide perioperative antibiotic prophylaxis in patients undergoing high-risk or medium-risk surgery (Tables 3, 4). (25) Two-dimensional echocardiography also delineates other structural abnormalities such as LV hypertrophy, systolic dysfunction, and/or other valvular disease, which may further change the perioperative/postoperative risk during noncardiac surgery. Aortic stenosis is accurately quantified by continuous-wave Doppler echocardiography in patients with preserved LV function by using the pressure gradient across the aortic valve (ie, the mean pressure gradient and the maximum instantaneous gradient). The aortic valve area can be determined by planimetry and can be calculated using the continuity equation. (30)
PREOPERATIVE CONSIDERATIONS OF THE ANESTHESIOLOGIST
Effective communication of the particular valvular lesion and its severity to the anesthesiologist and to the members of the surgical team is necessary to tailor perioperative care. As already stated for cardiovascular adverse events in noncardiac surgery, the type of surgery (Table 1) influences outcome in affected patients with aortic stenosis. (6) Therefore, it is of major importance to score the type of surgery and the physical exercise capacity of the patients into three classes, as has been suggested. (6) A good physical status (class 1) presumably enables the patient to undergo any surgical procedure with low risk despite severe aortic stenosis. In contrast, the postponing of elective noncardiac high-risk surgery may be advisable in patients with recent cardiac symptoms/decompensation (class 3) and aortic valve replacement before noncardiac surgery may be advisable (Fig 1). If this is not possible or if there can be no further delays in surgery, patients will be at high risk for a cardiac event during noncardiac surgery.
The group of patients with a mean transvalvular pressure gradient < 50 mm Hg and a fair physical status are at intermediate risk (class 2) and will usually show no problems during low-to-medium-risk procedures. However, the rate of complications increases in higher risk surgery. To our knowledge, the perioperative risk of patients with aortic stenosis and a moderately or severely depressed LV ejection fraction has not been systematically examined. The overall prognosis of patients with severe aortic stenosis and reduced LV ejection fraction is reduced (11,21) and is comparable to that of CAD patients with reduced LV function. The perioperative risk of former patients with aortic stenosis during noncardiac surgery is presumably high.
MANAGEMENT OF ANESTHESIA IN PATIENTS WITH AORTIC STENOSIS DURING NONCARDIAC SURGERY
The chronic pressure overload state induced by aortic stenosis results in concentric hypertrophy, subsequently reducing the compliance of the LV. Thus, ventricular filling is more dependent on pre-load and the functioning of the ventricular filling by the atria. It is not surprising that sinus tachycardia or atrial arrhythinias can worsen LV load, leading to heart failure in patients with aortic stenosis. In addition, concentric hypertrophy results in reduced coronary reserve. Thus, decreases in systemic vascular resistance may result in myocardial ischemia due to systemic hypotension and reduced coronary perfusion. In conclusion, the maintenance of normal sinus rhythm, adequate systemic resistance, and vascular volume are mandatory during anesthesia in patients with severe aortic stenosis. An adequate volume load is probably better than hypovolemia, and systemic hypotension should be aggressively treated preferably with [alpha]-adrenergic agonists. (31-33) In patients with aortic stenosis and insufficiency, the anesthesiologic management should be focused on the leading cause of aortic valve dysfunction.
Patients undergoing surgical procedures that induce an increase in the abdominal pressure (eg, laparoscopic procedures) or a quick and intense increase in the systemic BP (eg, cross-clamping maneuvers in vascular surgery) are at the highest risk for a perioperative cardiac event (Table 1). Therefore, a sufficient depth of general anesthesia, preferably opioid-based, is mandatory to avoid BP peaks and tachycardia. Epidural catheters employing local anesthetics with or without opioids play an important role in postoperative pain management, taking a reduced systemic vascular resistance into account, which should be intraoperatively treated with [alpha]-adrenergic agonists. Although the results of randomized controlled studies investigating anesthetic care in patients with aortic stenosis are lacking, beneficial effects may be expected.
Large clinical studies with adequate power are lacking that address specific anesthetic monitoring practice in patients with aortic stenosis during noncardiac surgery. ECG monitoring appears to be the simplest and most cost-effective way to control for sinus rhythm. The heart rate should be maintained at 50 to 60 beats/min in patients with aortic stenosis during anesthesia, while higher heart rates are allowed in patients with aortic stenosis who have higher degrees of aortic insufficiency. Arterial BP should be monitored invasively to maintain a diastolic pressure of about 60 mm Hg for sufficient coronary perfusion and a peak systolic ventricular pressure (ie, systolic arterial pressure plus peak pressure gradient) of < 200 mm Hg. Central venous cannulation should be performed carefully to avoid cardiac arrhythmias, but it is necessary to control the volume status to guarantee adequate end-diastolic filling of the heart. Despite the widespread use of pulmonary artery catheterization, the benefits of this intervention on patient outcome, even in patients undergoing high-risk noncardiac surgery, have never been convincingly demonstrated and should not be routinely applied. Pulmonary artery catheters may even increase the risk for pulmonary embolism and arrhythmias. (34) The routine use of intraoperative transesophageal echocardiography in patients with aortic stenosis undergoing noncardiac surgery is desirable, although its value is currently undetermined. Positive end-expiratory pressure ventilation will assist ventricular work but should be gradually introduced and carefully terminated.
The risk of mortality associated with anesthesia and noncardiac surgery has decreased from the 1950s to today despite the fact that more complex surgical procedures are being performed in sicker patients. However, this should not imply that patients with severe aortic stenosis will undergo a noncardiac surgery in a harmless manner because the presence of severe aortic stenosis imposes an increased risk that is associated with general and regional anesthesia. Nonetheless, the published data suggest that noncardiac procedures can be performed with reasonable safety in selected circumstances with close perioperative and postoperative monitoring.
Role of Valvuloplasty in Urgent Noncardiac Surgery
The value of preoperative balloon valvuloplasty in urgent noncardiac surgery in patients with aortic stenosis is a matter of discussion. Complications such as stroke, acute aortic insufficiency, and myocardial infarction occur in approximately 10 to 20% of patients and, thus, may limit its application before noncardiac surgery. Two small studies (35,36) have examined the role of balloon valvuloplasty prior to noncardiac surgery in seven patients needing urgent noncardiac surgery. No deaths, strokes, or other major adverse cardiac events were observed. (35,36) However, four major adverse cardiac events including one death were observed (37) in 15 patients with symptomatic aortic stenosis undergoing noncardiac surgery following balloon valvuloplasty of the aortic valve. No adverse events were observed in the 14 patients during noncardiac surgery who survived the valvuloplasty. (37) In summary, due to possible restenosis and clinical deterioration within a few months after undergoing valvuloplasty, balloon valvulotomy may play only a limited role in the treatment of patients who are not candidates for immediate valve replacement or who require urgent noncardiac surgery.
ANTIMICROBIAL PROPHYLAXIS IN PATIENTS WITH AORTIC STENOSIS
Antimicrobial prophylaxis for bacterial endocarditis has become standard and routine in most developed countries for patients who are at increased risk for bacterial endocarditis. (21,25) Although the biological plausibility is very high, no study has definitively demonstrated that prophylactic antibiotics prevent endocarditis after invasive procedures. Patients with aortic stenosis are considered to be at moderate risk for bacterial endocarditis and should therefore be offered antibiotic prophylaxis during surgery. (25) The types of surgery requiring antimicrobial prophylaxis and the recommended regimens are displayed in Tables 3 and 4.
SUMMARY
Severe aortic stenosis has been identified as an independent, important risk factor for patients undergoing noncardiac surgery. Some elegant studies have clearly demonstrated that the taking of a medical history and performance of a physical examination are the cornerstones of preoperative risk assessment, while technical tests add further independent information on the perioperative risk only in selected patient groups. Since perioperative risk is increased in patients with severe aortic stenosis (ie, a mean transvalvular gradient of > 50 mm Hg or a calculated aortic valve area of [less than or equal to] 1.0 [cm.sup.2]), (14) aortic valve replacement is recommended in affected patients with or without symptoms before noncardiac surgery. (6) The value of preoperative balloon angioplasty has not been determined and cannot be routinely recommended. In patients with mild-to-moderate aortic stenosis and a low-to-intermediate risk for CAD, perioperative treatment with [beta]-blockers may be prudent. If a high risk for CAD is present, coronary angiography and, eventually, revascularization should be considered. Since aortic stenosis is often accompanied by carotid artery disease, preoperative assessment regarding cerebrovascular risk and related treatment is mandatory. If urgent or emergent noncardiac surgery is imminent, perioperative risk may be reduced by a preoperative echocardiographic study, which helps to communicate the severity of the aortic stenosis to the anesthesiologist/surgeon to plan perioperative and postoperative care.
Manuscript received November 18, 2004; revision accepted April 19, 2005.
REFERENCES
(1) Mangano DT, Goldman L. Preoperative assessment of patients with known or suspected coronary disease. N Engl J Med 1995; 333:1750-1756
(2) Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med 1977; 297:845-850
(3) Fleisher LA, Eagle KA. Clinical practice: lowering cardiac risk in noncardiac surgery. N Engl J Med 2001; 345:1677-1682
(4) Detsky AS, Abrams HB, McLaughlin JR, et al. Predicting cardiac complications in patients undergoing non-cardiac surgery. J Gen Intern Med 1986; 1:211-219
(5) Schein OD, Katz J, Bass EB, et al. The value of routine preoperative medical testing before cataract surgery. Am J Ophthalmol 2000; 129:701
(6) Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery: executive summary; a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Anesth Analg 2002; 94:1052-1064
(7) Geldner G, Christ M, Wulf H. Preoperative assessment. Lancet 2004; 363:400-401
(8) Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease: Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630-634
(9) Lindroos M, Kupari M, Heikkila J, et al. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993; 21:1220-1225
(10) Lombard JT, Selzer A. Valvular aortic stenosis: a clinical and hemodynamic profile of patients. Ann Intern Med 1987; 106:292-298
(11) Carabello BA. Clinical practice: aortic stenosis. N Engl J Med 2002; 346:677-682
(12) Kertai MD, Bountioukos M, Boersma E, et al. Aortic stenosis: an underestimated risk factor for perioperative complications in patients undergoing noncardiac surgery. Am J Med 2004; 116:8-13
(13) Rohde LE, Polanczyk CA, Goldman L, et al. Usefulness of transthoracic echocardiography as a tool for risk stratification of patients undergoing major noncardiac surgery. Am J Cardiol 2001; 87:505-509
(14) O'Keefe JH Jr., Shub C, Rettke SR. Risk of noncardiac surgical procedures in patients with aortic stenosis. Mayo Clin Proc 1989; 64:400-40,5
(15) Torsher LC, Shub C, Rettke SR, et al. Risk of patients with severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol 1998; 81:448-452
(16) Sethi GK, Miller DC, Souchek J, et al. Clinical, hemodynamic, and angiographic predictors of operative mortality in patients undergoing single valve replacement: Veterans Administration Cooperative Study on Valvular Heart Disease. J Thorac Cardiovasc Surg 1987; 93:884-897
(17) Mullany CJ, Elveback LR, Frye RL, et al. Coronary artery disease and its management: influence on survival in patients undergoing aortic valve replacement. J Am Coll Cardiol (1987); 10:66-72
(18) Levinson JR, Akins CW, Buckley MJ, et al. Octogenarians with aortic stenosis: outcome after aortic valve replacement. Circulation 1989; 80:149-56
(19) Carabello BA. Aortic sclerosis: a window to the coronary arteries? N Engl J Med 1999; 341:193-195
(20) Otto CM, Lind BK, Kitzman DW, et al. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999; 341:142-147
(21) Bonow RO, Carabello B, de Leon AC, et al. ACC/AHA guidelines for the management of patients with valvular heart disease: executive summary; a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease). J Heart Valve Dis 1998; 7:672-707
(22) Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis: clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997; 95:2262-2270
(23) Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery: Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713-1720
(24) Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119:132S-175S
(25) Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis: recommendations by the American Heart Association. JAMA 1997; 277:1794-1801
(26) Devereaux PJ, Ghali WA, Gibson NE, et al. Physicians' recommendations for patients who undergo noncardiac surgery. Clin Invest Med 2000; 23:116-123
(27) Mollema R, Berger P, Girbes AR. The value of peri-operative consultation on a general surgical ward by the internist. Neth J Med 2000; 56:7-11
(28) Macpherson DS, Parenti C, Nee J, et al. An internist joins the surgery service: does comanagement make a difference? J Gen Intern Med 1994; 9:440-444
(29) Lee T, Pappius EM, Goldman L. Impact of inter-physician communication on the effectiveness of medical consultations. Am J Med 1983; 74:106-112
(30) Mochizuki Y, Pandian NG. Role of echocardiography in the diagnosis and treatment of patients with aortic stenosis. Curr Opin Cardiol 2003; 18:327-333
(31) Thomas SJ, Lowenstein E. Anesthetic management of the patient with valvular heart disease. Int Anesthesiol Clin 1979; 17:67-96
(32) Goertz AW, Lindner KH, Schutz W, et al. Influence of phenylephrine bolus administration on left ventricular filling dynamics in patients with coronary artery disease and patients with valvular aortic stenosis. Anesthesiology 1994; 81:49-58
(33) Goertz AW, Lindner KH, Seefelder C, et al. Effect of phenylephrine bolus administration on global left ventricular function in patients with coronary artery disease and patients with valvular aortic stenosis. Anesthesiology 1993; 78:834-841
(34) Sandham JD, Hull RD, Brant RF, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 2003; 348:5-14
(35) Levine MJ, Berman AD, Safian RD, et al. Palliation of preparation for noncardiac surgery. Am J Cardiol 1988; 62:1309-1310
(36) Roth RB, Palacios IF, Block PC. Percutaneous aortic balloon valvuloplasty: its role in the management of patients with aortic stenosis requiring major noncardiac surgery. J Am Coll Cardiol 1989; 13:1039-1041
(37) Hayes SN, Holmes DR Jr, Nishimura RA, et al. Palliative percutaneous aortic balloon valvuloplasty before noncardiac operations and invasive diagnostic procedures. Mayo Clin Proc 1989; 64:753-757
(38) Raymer K, Yang H. Patients with aortic stenosis: cardiac complications in non-cardiac surgery. Can J Anaesth 1998; 45:855-859
(39) Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043-1049
Michael Christ, MD; Yulia Sharkova, MD; Gotz Geldner, MD; and Bernhard Maisch, MD
* From the Departments of Internal Medicine and Cardiology (Drs. Christ, Sharkova, and Maisch) and Anesthesiology and Intensive Care (Dr. Geldner), Philipps University Marburg, Germany.
Dr. Sharkova received a grant from the Deutsche Herzstiftung, Frankfurt, Germany.
Correspondence to: Michael Christ, MD, Department Medizin, Med. Klinik A, Universitgitsspitals Basel, Basel, Switzerland; e-mail: christ_michael@yahoo.de
COPYRIGHT 2005 American College of Chest Physicians
COPYRIGHT 2005 Gale Group