Abstract
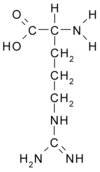
There is abundant evidence that the endothelium plays a crucial role in the maintenance of vascular tone and structure. One of the major endothelium-derived vasoactive mediators is nitric oxide (NO), an endogenous messenger molecule formed in healthy vascular endothelium from the amino acid precursor L-arginine. Endothelial dysfunction is caused by various cardiovascular risk factors, metabolic diseases, and systemic or local inflammation. One mechanism that explains the occurrence of endothelial dysfunction is the presence of elevated blood levels of asymmetric dimethylarginine (ADMA)--an L-arginine analogue that inhibits NO formation and thereby can impair vascular function. Supplementation with L-arginine has been shown to restore vascular function and to improve the clinical symptoms of various diseases associated with vascular dysfunction.
(Altern Med Rev 2005;10(1):14-23)
Introduction
The endothelium plays a crucial role in the maintenance of vascular tone and structure. One endothelium-derived vasoactive mediator with major importance is nitric oxide (NO), which is formed from the amino acid precursor L-arginine by the enzyme endothelial nitric oxide synthase (eNOS). NO is involved in a wide variety of regulatory mechanisms of the cardiovascular system, including vascular tone (it is the major mediator of endothelium-dependent vasodilation), vascular structure (inhibition of smooth muscle cell proliferation), and cell-cell interactions in blood vessels (inhibition of platelet adhesion and aggregation; inhibition of monocyte adhesion).
Dysfunction of the endothelial L-arginine/ nitric oxide pathway is a common mechanism by which several cardiovascular risk factors mediate certain deleterious effects on the vascular wall. Among these are hypercholesterolemia, hypertension, smoking, diabetes mellitus, homocysteine, and vascular inflammation? (1-6)
Supplementation with L-arginine in animals with experimentally-induced vascular dysfunction atherosclerosis improves endothelium-dependent vasodilation. (7-10) Moreover, L-arginine supplementation results in enhanced endothelium-dependent inhibition of platelet aggregation, inhibition of monocyte adhesion, and reduced vascular smooth muscle proliferation. (11-13) The mechanism by which dietary L-arginine brings about these beneficial effects has long been poorly understood. Experimental evidence derived from studying cloned, purified eNOS in a cell-free system in vitro, and in the presence of optimal concentrations of all co-factors, suggests L-arginine concentrations as low as 3 [micro]mol/L are sufficient to induce half-maximal activity of this enzyme. (14) In contrast, circulating L-arginine measured in plasma of healthy humans as well as in plasma of patients with vascular disease is in the range of 40-100 [micro]mol/L (15,16)--which is 15- to 30-fold higher.
ADMA is a Novel Cardiovascular Risk Factor
In 1992, Vallance et al first described the presence of asymmetric dimethylarginine (ADMA) as an endogenous inhibitor of eNOS in human plasma and urine. (17) Since then, the role of this molecule in the regulation of eNOS has attracted increasing attention. ADMA inhibits vascular NO production within the concentration range found in patients with vascular disease. ADMA also causes local vasoconstriction when infused intra-arterially, and increases systemic vascular resistance and impairs renal function when infused systemically. Currently available experimental and clinical evidence suggests even small modifications of ADMA levels significantly change vascular NO production, vascular tone, and systemic vascular resistance (for review, see Boger (18). Thus, elevated ADMA levels may explain the "L-arginine paradox;" i.e., the observation that supplementation with exogenous L-arginine improves NO-mediated vascular functions in vivo, although its baseline plasma concentration is about 25-fold higher than the Michaelis Constant (Km) of the isolated, purified endothelial NO synthase in vitro (Figure 1).
[FIGURE 1 OMITTED]
Elevated ADMA concentration has a high prevalence in hypercholesterolemia, hyperhomocysteinemia, diabetes mellitus, peripheral arterial occlusive disease, hypertension, chronic heart failure, coronary artery disease, pregnancy-induced hypertension and preeclampsia, erectile dysfunction, and other clinical conditions (Table 1). (1,19-28)
Several recent studies have supplied evidence to support a pathophysiological role of ADMA in the pathogenesis of vascular dysfunction and cardiovascular disease. High ADMA levels were found to be associated with carotid artery intima-media-thickness in a study with 116 clinically healthy human subjects. (29) Taking this observation further, another study performed with hemodialysis patients reported that ADMA prospectively predicted the progression of intimal thickening during one year of follow-up. (30) In a nested, case-control study involving 150 middle-aged, non-smoking men, high ADMA levels were associated with a 3.9-fold elevated risk for acute coronary events. (31)
In the first prospective clinical trial, ADMA was determined to be the strongest predictor of cardiovascular events and total mortality in 225 hemodialysis patients during three years of follow-up. Patients whose ADMA levels were within the highest quartile at the beginning of the study had a three-fold higher risk of death from any cause than patients with ADMA levels below the median. (25) Another study investigated factors related to outcome of patients undergoing intensive care unit treatment for multiple causes. Patients whose ADMA levels were in the highest quartile had a 17-fold increase in mortality compared to patients with ADMA levels in the lowest quartile. (32) In a third prospective study, the outcome of patients with stable angina pectoris after coronary balloon angioplasty was addressed, and patients with high ADMA levels were found to have an elevated risk of developing severe cardiovascular complications. (33) In each of these studies, other cardiovascular risk factors and confounding variables were included in the analyses, and ADMA was found to predict cardiovascular risk independent of other variables. Thus, it has recently been concluded that ADMA can be considered to be a novel cardiovascular risk factor. (34-36)
The Role of ADMA for Explaining the Beneficial
Effects of Nutritional L-Arginine Supplementation
Circulating L-arginine concentrations have been found to be within the normal range in most clinical conditions associated with endothelial dysfunction. Few patients experience pathologically low L-arginine concentrations. However, clinical and experimental evidence suggests elevation of ADMA can cause a relative L-arginine deficiency, even in the presence of "normal" L-arginine levels (which may, in fact, be too low in these conditions). As ADMA is a competitive inhibitor of eNOS, its inhibitory action can be overcome by increasing the concentration of the enzyme's substrate, L-arginine (Figure 2). The studies cited above indicate ADMA levels may be increased in conditions associated with cardiovascular diseases. Elevated ADMA concentration is one possible explanation for endothelial dysfunction and decreased NO production in these diseases. In this respect, the authors recently observed improved endothelium-dependent vasodilation after L-arginine administration in patients with congestive heart failure (who had elevated ADMA concentrations); whereas, L-arginine did not affect endothelium-dependent vasodilation in healthy human subjects (who had low ADMA concentrations). (37) Thus, nutritional supplementation with L-arginine may help to restore the physiological status by normalizing the L-arginine/ ADMA ratio; whereas, its effects are less pronounced in humans without a disturbed L-arginine/ADMA balance. Normally, L-arginine/ADMA ratio is in the range of 50:1 to 100:1, given a range of L-arginine levels between 50 and 100 umol/L, and ADMA concentrations between 0.3 and 0.7 [micro]mol/L.
A beneficial effect of L-arginine on vascular function has been found by several different groups of investigators in patients with impaired vascular function (see below); whereas, little or no effect is usually noted in healthy controls. (16) This makes sense, as the molecular function of L-arginine, as detailed above, is to restore endothelial NO production to normal, thereby normalizing vascular function. By replenishing eNOS with its natural substrate, no vasodilator effects beyond the physiological range can be expected. Thus, no exaggerated hypotensive action, orthostatic dysregulation, or adverse cardiac events related to reflex tachycardia need be considered. In contrast to L-arginine, exogenous NO donors such as the organic nitrates, which release NO after enzymatic conversion by the activity of enzymes different from eNOS, are associated with tolerance development and oxidative stress to the arterial wall. (38) In this respect, there is ample evidence to consider L-arginine a safe and beneficial dietary supplement.
One other aspect in the vascular effects of ADMA and L-arginine may be of therapeutic relevance. The beneficial vascular effects of HMG-Co A reductase inhibiting, cholesterol-lowering drugs (statins) have been shown to partially be due to their ability to up-regulate eNOS gene expression. (39) However, statins failed to improve endothelium-dependent vasodilation in about 50 percent of studies that examined their effects on vasodilation. (40-43) This discrepancy may be resolved by examining ADMA. Janatuinen et al (44) recently found pravastatin enhanced myocardial blood flow, measured by positron electron resonance tomography (PET), in patients with low ADMA; however, the drug was ineffective in patients with elevated ADMA. The authors speculate that ADMA may block eNOS despite its up-regulated gene expression after statin treatment, and that this blockade may be overcome by L-arginine supplementation. (18) In a randomized, controlled trial of patients with elevated ADMA concentration, simvastatin enhanced endothelium-dependent vasodilation only when combined with a sustained-release L-arginine formulation. (45) Endothelium-dependent vasodilation was 5.5 [+ or -] 0.5 percent at baseline in this study, compared to an expected 8-12 percent in healthy humans. Simvastatin alone did not improve endothelium-mediated vasodilation (6.2 [+ or -] 1.2%); whereas, the combination of simvastatin with L-arginine significantly enhanced endothelium-dependent vasodilation (9.8 [+ or -] 1.5%).
Thus, it may be that ADMA levels explain the inconsistent results of clinical trials in which statin drugs improved endothelial function or failed to do so--a discrepancy so far unexplained. The observation of elevated ADMA concentration in a given patient may warrant supplementing with L-arginine in order to improve the ability of the endothelium to counteract offenses to the vascular wall by circulating blood cells, vasoconstrictors, and oxygen-derived free radicals.
Beneficial Effects of Supplemental L-Arginine on Vascular Function Angina
Arginine supplementation has been effective in the treatment of cardiovascular dysfunction. In an uncontrolled trial, seven of 10 people with intractable angina showed significant improvement after taking 9 g arginine daily for three months. (46) Significant decreases in cell adhesion molecules and pro-inflammatory cytokine levels were also observed. A double-blind trial in 22 patients with stable angina and healed myocardial infarction showed oral supplementation with 6 g arginine daily for three days increased exercise capacity. (47)
In men with stable angina, two weeks of supplementation with arginine (15 g per day) was not associated with improvement in endothelium-dependent vasodilation, oxidative stress, or exercise performance. (48) In patients with coronary artery disease, oral supplementation of arginine (6 g per day for three days) did not affect exercise-induced changes in QT-interval duration, QT dispersion, or the magnitude of ST-segment depression; (49) however, it did significantly increase exercise tolerance. The therapeutic effect of arginine in patients with microvascular angina is considered to be the result of improved endothelium-dependent coronary vasodilation. (50)
Congestive Heart Failure
Patients with congestive heart failure (CHF) have reduced peripheral blood flow at rest, during exercise, and in response to endothelium-dependent vasodilators. Nitric oxide formed from arginine metabolism in endothelial cells can contribute to regulation of blood flow under these conditions. A randomized, double-blind trial (51) found six weeks of arginine supplementation (5.6-12.6 g per day) significantly improved blood flow, arterial compliance, and functional status compared to placebo. Another double-blind trial found arginine supplementation (5 g three times per day) improved renal function in individuals with CHF. (52)
Hypertension
Hypertension is a major healthcare problem afflicting nearly 50 million people in the United States. (53) Despite its strong causal association with cardiovascular disease complications, including myocardial infarction, heart failure, and stroke, the majority of patients with hypertension do not achieve optimal blood pressure control. The prevalence of hypertension is expected to increase with the aging population, growing obesity, and rising incidence of metabolic syndrome.
Endothelial dysfunction and reduced NO bioactivity represent prominent pathophysiological abnormalities associated with hypertensive cardiovascular disease. Individuals with hypertension exhibit blunted epicardial and resistance vascular dilation to NO in the peripheral and coronary circulation that likely contributes to mechanisms of altered vascular tone in hypertension. L-arginine has been shown to reduce systemic blood pressure in some forms of experimental hypertension. (54)
Erectile Dysfunction
Erectile dysfunction (ED) is defined as the persistent inability to attain and maintain an erection sufficient to permit satisfactory sexual intercourse. According to the National Institutes of Health, ED has been reported to affect as many as 20-30 million men in the United States and 152 million men worldwide. The risk for ED increases progressively with advancing age, with an estimated 54 percent of men ages 65-70 reporting some degree of impotence. (55) It is believed 85-90 percent of ED cases are related to a physical or medical condition, while 10-15 percent are due to psychological causes.
Erectile dysfunction is an important part of the total clinical picture in primary care, not only for its psychosocial significance, but also as a possible early indicator of general vascular compromise. (55) ED can be an early indicator of cardiovascular disease, caused by an underlying dysfunction of the arteries and vascular system. ED is commonly associated with a number of conditions frequently occurring in aging men, including prostatic hypertrophy, ischemic heart disease, peripheral vascular disease, hypertension, atherosclerosis, hyperlipidemia, stroke, and diabetes mellitus.
In a group of 15 men with ED, six in the group taking 2.8 g arginine daily for two weeks experience benefit, compared to no improvement in the placebo group. Although little is known about how effective arginine will be for men with erectile dysfunction or which subset of men would most likely be helped, available research looks promising and suggests that at least some men will benefit. (56) In a controlled clinical trial, 50 patients with ED were treated with 5 g L-arginine daily or placebo for six weeks. Nine of 29 patients taking L-arginine (31%), but only two of 17 patients taking placebo (11.7%), reported significant improvement of sexual function. In the nine responders, significant increases in plasma and urinary nitrate were measured after treatment with L-arginine, indicating improved NO production secondary to this treatment. (57)
Sickle Cell Disease and Pulmonary Hypertension
Pulmonary hypertension is a life-threatening complication of sickle cell disease reported to occur in up to 30 percent of adults with this disease. The etiology of sickle cell disease-related pulmonary hypertension is unclear. Treatment options are limited, and the prognosis is poor. Patients who develop this complication have a shortened lifespan. Its presence is an independent predictor of death, with an average time to death after diagnosis as short as 12 months.
Several studies in non-sickle cell disease patients demonstrate therapeutic benefits of L-arginine therapy for pulmonary hypertension. (58,59) Low plasma L-arginine concentrations have been discovered in infants with persistent pulmonary hypertension of the newborn, (60) correlating with low nitric oxide metabolite levels. (61) L-arginine infusion has decreased pulmonary vascular resistance and improved blood oxygenation in infants with this disease process. (62) L-arginine supplementation also improves pulmonary artery pressures and hemodynamics in patients with primary and secondary pulmonary hypertension, (59) with one recent study demonstrating these effects after only one week of therapy. (58)
Recent studies found that oral L-arginine normalizes red blood cell density and induces Gardos channel inhibition in sickle cell transgenic mice. (63) There is otherwise limited information available on the impact of L-arginine supplementation in sickle cell disease. (64)
There is growing evidence that pulmonary hypertension is a disease process that involves altered L-arginine metabolism or decreased bioavailability. Arginase, the enzyme that converts L-arginine to ornithine and urea, may limit NO bioavailability in sickle cell disease through increased use of its substrate. (65) In one recent study, L-arginine at a dose of 0.1 g/kg three times daily produced a 15.2-percent mean reduction in estimated pulmonary artery systolic pressure (63.9 [+ or -] 13 to 54.2 [+ or -] 12 mm Hg, p = 0.002) after five days of therapy in 10 patients. Arginase activity was elevated almost two-fold (p = 0.07) in patients with pulmonary hypertension and may limit L-arginine bioavailability. (65) Because L-arginine supplementation improves pulmonary artery pressures in non-sickle cell patients with pulmonary hypertension, L-arginine may also be therapeutic for sickle cell disease patients with pulmonary hypertension by providing increased substrate for NO production.
Beneficial Effects of Supplemental Sustained-release L-Arginine
From the above-mentioned studies of L-arginine, it appears an effective method of improving endothelial function would be to supplement with L-arginine. Oral L-arginine, however, is absorbed and metabolized quickly; the half-life of L-arginine in human circulating plasma is less than one hour. (66) A controlled-release formulation of L-arginine would increase the length of time in which L-arginine achieves an effective concentration.
Boger et al found 1.5 g of a sustained-release L-arginine taken twice daily improved endothelium-dependent vasodilation in patients with high plasma ADMA levels. (45) In previous studies, the same group of researchers showed at least 6 g L-arginine in a nonsustained release formulation was needed to achieve a similar effect. (67)
In a preliminary study of five patients with coronary artery disease in whose myocardial perfusion had been maximized and stabilized on conventional cardiovascular medications, 3 g sustained-release L-arginine was given twice daily for 12 weeks. Significant improvements in heart function and myocardial perfusion were seen via PET imaging. (68)
Conclusions
ADMA is an endogenous and competitive inhibitor of NO synthase. Plasma levels of this inhibitor are elevated in patients with atherosclerosis and in those with risk factors for atherosclerosis. (34,36) In these patients, plasma ADMA levels are correlated with the severity of endothelial dysfunction and atherosclerosis. By inhibiting the production of NO, ADMA can impair blood flow, accelerate atherogenesis, and interfere with angiogenesis.
Supplemental L-arginine improves endothelial function, myocardial perfusion, angina, erectile dysfunction, and exercise tolerance, regardless of ADMA status. However, many patients exhibiting one of these impairments demonstrate elevated blood ADMA. Therefore, testing for plasma ADMA levels may give the physician a better idea of those patients who may respond best to prolonged L-arginine supplementation, as data are accumulating to show that patients with elevated ADMA are the most likely to benefit. The ratio of L-arginine to ADMA is considered to be the most accurate measure of eNOS substrate availability. This ratio will increase during L-arginine supplementation, regardless of initial ADMA concentration. Due to the pharmacokinetics of oral L-arginine and the positive results from preliminary studies, it appears supplementation with a sustained-release L-arginine preparation will achieve positive therapeutic results at lower dosing levels.
References
(1.) Boger RH, Bode-Boger SM, Szuba A, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 1998;98:1842-1847.
(2.) Panza JA, Quyyumi AA, Brush JE Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 1990;323:22-27.
(3.) Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993;88:2149-2155.
(4.) Johnstone MT, Creager SJ, Scales KM, et al. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation 1993;88:2510-2516.
(5.) Tawakol A, Omland T, Gerhard M, et al. Hyperhomocyst(e)inemia is associated with impaired endothelium-dependent vasodilation in humans. Circulation 1997;95:1119-1121.
(6.) Hingorani AD, Cross J, Kharbanda RK, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation 2000; 102:994-999.
(7.) Cooke JP, Andon NA, Girerd XJ, et al. Arginine restores cholinergic relaxation of hypercholesterolemic rabbit thoracic aorta. Circulation 1991;83:1057-1062.
(8.) Boger RH, Bode-Boger SM, Mugge A, et al. Supplementation of hypercholesterolaemic rabbits with L-arginine reduces the vascular release of superoxide anions and restores NO production. Atherosclerosis 1995;117:273-284.
(9.) Candipan RC, Wang BY, Buitrago R, et al. Regression or progression. Dependency on vascular nitric oxide. Arterioscler Thromb Vasc Biol 1996;16:44-50.
(10.) Boger RH, Bode-Boger SM, Brandes RE et al. Dietary L-arginine reduces the progression of atherosclerosis in cholesterol-fed rabbits: comparison with lovastatin. Circulation 1997;96:1282-1290.
(11.) Tsao PS, Theilmeier G, Singer AH, et al. L-arginine attenuates platelet reactivity in hypercholesterolemic rabbits. Arterioscler Thromb 1994; 14:1529-1533.
(12.) Tsao PS, McEvoy LM, Drexler H, et al. Enhanced endothelial adhesiveness in hypercholesterolemia is attenuated by L-arginine. Circulation 1994;89:2176-2182.
(13.) Boger RH, Bode-Boger SM, Kienke S, et al. Dietary L-arginine decreases myointimal cell proliferation and vascular leukocyte accumulation in cholesterol-fed rabbits. Atherosclerosis 1998; 136:67-77.
(14.) Pollock JS, Forstermann U, Mitchell JA, et al. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A 1991;88:10480-10484.
(15.) Jeserich M, Munzel T, Just H, Drexler H. Reduced plasma L-arginine in hypercholesterolaemia. Lancet 1992;339:561.
(16.) Boger RH, Bode-Boger SM. The clinical pharmacology of L-arginine. Annu Rev Pharmacol Toxicol 2001;41:79-99.
(17.) Vallance P, Leone A, Calver A, et al. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol 1992;20:$60-$62.
(18.) Boger RH. Asymmetric dimethylarginine (ADMA) modulates endothelial function--therapeutic implications. Vasc Med 2003;8:149-151.
(19.) Lundman P, Eriksson MJ, Stuhlinger M, et al. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J Am Coll Cardiol 2001;38:111-116.
(20.) Surdacki A, Nowicki M, Sandmann J, et al. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J Cardiovasc Pharmacol 1999;33:652-658.
(21.) Gorenflo M, Zheng C, Werle E, et al. Plasma levels of asymmetrical dimethyl-L-arginine in patients with congenital heart disease and pulmonary hypertension. J Cardiovasc Pharmacol 2001;37:489-492.
(22.) Boger RH, Bode-Boger SM, Thiele W, et al. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation 1997;95:2068-2074.
(23.) Boger RH, Bode-Boger SM, Thiele W, et al. Restoring vascular nitric oxide formation by L-arginine improves the symptoms of intermittent claudication in patients with peripheral arterial occlusive disease. J Am Coll Cardiol 1998;32:1336-1344.
(24.) Kielstein JY, Boger RH, Bode-Boger SM, et al. Asymmetric dimethylarginine plasma concentrations differ in patients with end-stage renal disease: relationship to treatment method and atherosclerotic disease. J Am Soc Nephrol 1999;10:594-600.
(25.) Zoccali C, Bode-Boger SM, Mallamaci F, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet 2001; 358:2113-2117.
(26.) Usui M, Matsuoka H, Miyazaki H, et al. Increased endogenous nitric oxide synthase inhibitor in patients with congestive heart failure. Life Sci 1998;62:2425-2430.
(27.) Abbasi F, Asagmi T, Cooke JP, et al. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am J Cardiol 2001;88:1201-1203.
(28.) Pettersson A, Hedner T, Milsom I. Increased circulating concentrations of asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, in preeclampsia. Acta Obstet Gynecol Scand 1998;77:808-813.
(29.) Miyazaki H, Matsuoka H, Cooke JP. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation 1999;99:1141-1146.
(30.) Zoccali C, Benedetto FA, Maas R, et al. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. JAm Soc Nephrol 2002;13:490-496.
(31.) Valkonen VP, Paiva H, Salonen JY, et al. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet 2001;358:2127-2128.
(32.) Nijveldt RJ, Teerlink T, van der Hoven B, et al. Asymmetrical dimethylarginine (ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clin Nutr 2003;22:23-30.
(33.) Lu TM, Ding YA, Lin SJ, et al. Plasma levels of asymmetrical dimethylarginine and adverse cardiovascular events after percutaneous coronary intervention. Eur Heart J 2003 ;24:1912-1919.
(34.) Boger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res 2003;59:824-833.
(35.) Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol 2004;24:1023-1030.
(36.) Cooke JR Asymmetrical dimethylarginine: the Uber marker? Circulation 2004;109:1813-1818.
(37.) Hornig B, Arakawa N, Boger RH, et al. Plasma levels of ADMA are increased and inversely related to endothelium-mediated vasodilation in patients with chronic heart failure: a new predictor of endothelial dysfunction? Circulation 1998;98:I-318.
(38.) Munzel T, Sayegh H, Freeman BA, et al. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J Clin Invest 1995;95:187-194.
(39.) Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998;97:1129-1135.
(40.) Leung WH, Lau CE Wong CK. Beneficial effect of cholesterol-lowering therapy on coronary endothelium-dependent relaxation in hypercholesterolaemic patients. Lancet 1993;341:1496-1500.
(41.) Treasure CB, Klein JL, Weintraub WS, et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med 1995;332:481-487.
(42.) Vita JA, Yeung AC, Winniford M, et al. Effect of cholesterol-lowering therapy on coronary endothelial vasomotor function in patients with coronary artery disease. Circulation 2000; 102:846-851.
(43.) van Venrooij FV, van de Ree MA, Bots ML, et al. Aggressive lipid lowering does not improve endothelial function in type 2 diabetes: the Diabetes Atorvastatin Lipid Intervention (DALI) Study: a randomized, double-blind, placebo-controlled trial. Diabetes Care 2002;25:1211-1216.
(44.) Janatuinen T, Laakso J, Laaksonen R, et al. Plasma asymmetric dimethylarginine modifies the effect of pravastatin on myocardial blood flow in young adults. Vasc Med 2003;8:185-189.
(45.) Boger GI, Maas R, Schwedhelm E, et al. Improvement of endothelium-dependent vasodilation by simvastatin is potentiated by combination with L-arginine in patients with elevated asymmetric dimethylarginine levels. J Am Coil Cardiol 2004;Suppl:525A.
(46.) Blum A, Porat R, Rosenschein U, et al. Clinical and inflammatory effects of dietary L-arginine in patients with intractable angina pectoris. Am J Cardiol 1999;83:1488-1490.
(47.) Ceremuzynski L, Chamiec T, Herbaczynska-Cedro K. Effect of supplemental oral L-arginine on exercise capacity in patients with stable angina pectoris. Am J Cardiol 1997;80:331-333.
(48.) Walker HA, McGing E, Fisher I, et al. Endothelium-dependent vasodilation is independent of the plasma L-arginine/ADMA ratio in men with stable angina: lack of effect of oral L-arginine on endothelial function, oxidative stress and exercise performance. J Am Coll Cardiol 2001;38:499-505.
(49.) Bednarz B, Wolk R, Chamiec T, et al. Effects of oral L-arginine supplementation on exercise-induced QT dispersion and exercise tolerance in stable angina pectoris. Int J Cardiol 2000;75:205-210.
(50.) Egashira K, Hirooka Y, Kuga T, et al. Effects of L-arginine supplementation on endothelium-dependent coronary vasodilation in patients with angina pectoris and normal coronary arteriograms. Circulation 1996;94:130-134.
(51.) Rector TS, Bank AJ, Mullen KA, et al. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation 1996;93:2135-2141.
(52.) Watanabe G, Tomiyama H, Doba N. Effects of oral administration of L-arginine on renal function in patients with heart failure. J Hypertens 2000;18:229-234.
(53.) Gokce N. L-arginine and hypertension. J Nutr 2004;134:2807S-2811S; discussion 2818S-2819S.
(54.) Lim DS, Mooradian SJ, Goldberg CS, et al. Effect of oral L-arginine on oxidant stress, endothelial dysfunction, and systemic arterial pressure in young cardiac transplant recipients. Am J Cardiol 2004;94:828-831.
(55.) Nicolosi A, Moreira ED Jr, Shirai M, et al. Epidemiology of erectile dysfunction in four countries: cross-national study of the prevalence and correlates of erectile dysfunction. Urology 2003;61:201-206.
(56.) Zorgniotti AW, Lizza EE Effect of large doses of the nitric oxide precursor, L-arginine, on erectile dysfunction. Int J Impot Res 1994;6:33-35.
(57.) Chen J, Wollman Y, Chernichovsky T, et al. Effect of oral administration of high-dose nitric oxide donor L-arginine in men with organic erectile dysfunction: results of a double-blind, randomized, placebo-controlled study. BJU Int 1999;83:269-273.
(58.) Nagaya N, Uematsu M, Oya H, et al. Short-term oral administration of L-arginine improves hemodynamics and exercise capacity in patients with precapillary pulmonary hypertension. Am J Respir Crit Care Med 2001;163:887-891.
(59.) Mehta S, Stewart DJ, Langleben D, Levy RD. Short-term pulmonary vasodilation with L-arginine in pulmonary hypertension. Circulation 1995 ;92:1539-1545.
(60.) Vosatka RJ, Kashyap S, Trifiletti RR. Arginine deficiency accompanies persistent pulmonary hypertension of the newborn. Biol Neonate 1994;66:65-70.
(61.) Pearson DL, Dawling S, Walsh WF, et al. Neonatal pulmonary hypertension--urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med 2001;344:1832-1838.
(62.) McCaffrey MJ, Bose CC, Reiter PD, Stiles AD. Effect of L-arginine infusion on infants with persistent pulmonary hypertension of the newborn. Biol Neonate 1995;67:240-243.
(63.) Romero JR, Suzuka SM, Nagel RL, Fabry ME. Arginine supplementation of sickle transgenic mice reduces red cell density and Gardos channel activity. Blood 2002;99:1103-1108.
(64.) Morris CR, Kuypers FA, Larkin S, et al. Arginine therapy: a novel strategy to induce nitric oxide production in sickle cell disease. Br J Haematol 2000; 111:498-500.
(65.) Morris CR, Morris SM Jr, Hagar W, et al. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med 2003;168:63-69.
(66.) Bode-Boger SM, Boger RH, Galland A, et al. L-arginine-induced vasodilation in healthy humans: pharmacokinetic-pharmacodynamic relationship. Br J Clin Pharmacol 1998;46:489-497.
(67.) Sydow K, Schwedhelm E, Arakawa N, et al. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc Res 2003;57:244-252.
(68.) Ron ES, Gould KL. Unpublished results.
Rainer H. Boger, MD--Professor and Head, Clinical Pharmacology Unit, Institute of Experimental and Clinical Pharmacology, Center of Experimental Medicine, University Hospital Hamburg-Eppendorf, Germany. Correspondence address: Martinistr. 52, D-20246 Hamburg, Germany. E-mail: boeger@uke.uni-hamburg.de
Eyal S. Run, PhD--Chief Scientific Officer, eNOS Pharmaceuticals, Inc., Cambridge, MA. eNOS Pharmaceuticals is involved in research and development of time-release arginine.
COPYRIGHT 2005 Thorne Research Inc.
COPYRIGHT 2005 Gale Group