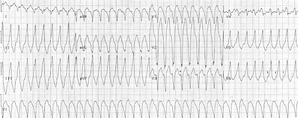
A 31-year-old white man collapsed suddenly at a graduation ceremony and was pronounced dead after attempted resuscitation. He had no pertinent medical or familial history. Postmortem toxicologic studies showed negative results. A complete autopsy revealed a cardiac cause of death. Grossly, the right ventricular chamber was moderately to markedly dilated, and its free wall showed extensive myocardial adiposity. Microscopically, the right ventricular free wall consisted predominantly of adipose tissue, with only small subendocardial islands of hypertrophied myocytes and interstitial fibrosis. These features are characteristic of arrhythmogenic right ventricular cardiomyopathy. Moreover, Purkinje-like cells were observed among right ventricular myocytes and may have increased the likelihood of developing an arrhythmia. To our knowledge, this finding has not been previously emphasized. Because arrhythmogenic right ventricular cardiomyopathy accounts for 10% of cases of sudden unexpected cardiac death, recognition of this disease by pathologists is important, especially in cases of otherwise unexplained death in young persons.
(Arch Pathol Lab Med. 2005;129:1330-1333)
Arrhythmogenic right ventricular cardiomyopathy (ARVCM), also known as arrhythmogenic right ventricular dysplasia, is an uncommon disorder characterized by right ventricular dilatation and fibrofatty replacement of the right ventricular myocardium. Although it has been estimated that the disease afflicts about 1 in 5000 persons in the United States, the exact prevalence is unknown.1
In 80% of the cases, the initial manifestation occurs before the age of 40 years and includes palpitations, presyncope or syncope, transient or sustained ventricular tachycardia, cardiac arrest, congestive heart failure, or sudden unexpected death.1-3 In many cases of sudden death, there is no prior history of an underlying cardiac disorder.
We present a case with typical gross and microscopic features of ARVCM, as well as a unique microscopic finding. Because the deceased had no previous family history or related medical history, the disease was undiagnosed until autopsy.
REPORT OF A case
A 31-year-old white man collapsed suddenly while attending his son's graduation ceremony in May 2004. He was pronounced dead after unsuccessful attempted resuscitation. Postmortem toxicologie studies showed negative results for alcohol or other drug abuse.
He had no history of heart disease, and there was no history of recent travel, infection, or illness. His family history included a cranial aneurysm in a paternal uncle, but there was no history of arrhythmia, cardiomyopathy, or unexpected sudden death.
AUTOPSY FINDINGS
A complete autopsy was performed. A well-developed, nonobese, white man showed no external injuries, and his non thoracic organs were within normal limits. Both lungs were mildly congested (450-g right lung and 350-g left lung). Grossly, there were no pulmonary emboli, and microscopically there were no features of pulmonary hypertension.
Gross Cardiac Features
Although the heart weight of 369 g was within the normal range, the right ventricular chamber was moderately to markedly dilated (Figure Y), had an internal short-axis diameter of 5.5 cm, and showed apical extension beyond that of the left ventricle. Moreover, the right ventricular free wall showed extensive replacement of the myocardium by adipose tissue (Figure 2). Its remaining myocardial muscle, excluding trabeculations, ranged from only 0.1 to 0.4 cm in thickness (expected thickness, 0.3-0.5 cm). Aneurysmal dilatation occurred along the inferior wall of the right ventricle.
The right atrial chamber and all 4 valve rings were mildly dilated. In contrast, the left ventricle was of normal gross appearance and thickness (1.3-cm free wall and 1.5cm septum). Epicardial coronary arteries showed no anomalies and only minimal focal atherosclerosis, and there were no old or recent myocardial infarcts. Valves showed no vegetations, and cardiac chambers contained no mural thrombi.
Microscopic Cardiac Features
The right ventricular free wall displayed numerous areas with nearly transmural adiposity (Figure 3). In these areas, the subendocardial region contained small focal strips or clusters of hypertrophied myocytes, focally vacuolated myocytes, and large pale-staining Purkinje-like cells, with focal pericellular fibrosis (Figure 4). Right ventricular trabeculations, although lacking adiposity, showed focal areas of pericellular or replacement fibrosis, as well as hypertrophied myocytes and Purkinje-like cells.
To further characterize the Purkinje-like cells, numerous immunohistochemical stains were performed. These cells reacted positively with antibodies directed against desmin and myoglobin, as well as weakly positively for actin and α^sub 1^-antitrypsin. They were negative for vimentin, CAM 5.2, SlOO, and lysozyme. Transmission electron microscopy was also attempted, but the quality of material processed from paraffin-embedded tissue was suboptimal for ultrastructural analysis. Nevertheless, the immunohistochemical findings support the myocyte and Purkinje cell origin of the observed right ventricular cells, although the weakly positive results with antibodies for α^sub 1^-antitrypsin are difficult to explain.
The left ventricle displayed only a few microfocal areas of adiposity, which were localized to the subepicardial region. Neither ventricle contained foci of myocyte necrosis or apoptosis, inflammatory infiltrates, active myocarditis, or viral inclusions.
COMMENT
The present case illustrates several classic features of ARVCM. The 5 following topics warrant emphasis and discussion: (1) definition and demographics; (2) pathogenesis and genetics; (3) gross pathology; (4) microscopic pathology, including the observation of Purkinje-like cells; and (5) association with sudden death.
Definition and Demographics
Arrhythmogenic right ventricular cardiomyopathy is characterized by myocyte loss due to necrosis or apoptosis, as well as fatty or fibrofatty replacement.1-3 It primarily affects the right ventricular free wall and may be focal or diffuse. The disease tends to progress from subepicardium to subendocardium and is eventually associated with wall thinning, focal aneurysm formation, and chamber dilatation.
Although ARVCM may be diagnosed at any age, sudden death tends to occur between the ages of 15 and 45 years, with a mean age of about 30 years.4-6 Men are affected slightly more often than women. In a series of 200 cases reported by Tabib et al,6 the mean age was 34 years (range, 5-65 years), and 108 (54%) were male. The present case of sudden death affected a 31-year-old man.
Pathogenesis and Genetics
The development of ARVCM appears to be related to the following 2 processes: (1) myocyte degeneration (including apoptosis and transdifferentiation), which may be inherited, and (2) interstitial inflammation, which may be infectious (probably postviral) or autoimmune in origin.1-3,7,8 Both processes may be operative in some patients. Fatty infiltration, which is the hallmark of the disorder, is considered to represent a secondary phenomenon.
Arrhythmogenic right ventricular cardiomyopathy is familial in at least 25% of the cases and in perhaps as many as 50%.c| There was no family history of ARVCM or sudden death in the present case. In most affected families, the disorder is transmitted as an autosomal dominant trait with variable penetrance and incomplete expression. Linkage analysis indicates that ARVCM is a genetically heterogeneous disorder, but in only about half of the patients can chromosomal defects be identified.
For the autosomal dominant form, defects have been localized to Iq42-q43 (ARVD2), 2q32 (ARVD4), 3p23 (ARVDS), 10q22 (ARVD7), 10pl2-pl4 (ARVD6), UqUq22 (ARVD3), and 14q23-q24 (ARVDl).10,11 Corresponding gene mutations have recently been identified and suggest an underlying ion channel disorder.11 A rare autosomal recessive form of ARVCM, occurring with Naxos disease (skin abnormalities and woolly hair), has been associated with defects at 6p24 and 17q21.1(1-12
Gross Pathology
In this disorder, hearts are normal in size or only mildly enlarged in weight or volume.4-'1 The primary feature of ARVCM is focal or diffuse replacement of right ventricular myocardium by adipose tissue, with a predilection for the infundibular, apical, and posteroinferior regions (socalled triangle of dysplasia).3 Fatty infiltration is nearly transmural and accompanied by thinning of the muscular wall, as seen in the present case.3 Thinned areas form focal aneurysms in about half of the hearts.3 Grossly, fatty infiltration generally spares the right ventricular trabeculations, the ventricular septum, and the left ventricular free wall.5
Microscopic Pathology
Arrhythmogenic right ventricular cardiomyopathy is characterized microscopically by replacement of portions of the right ventricular free wall by adipose tissue that is in excess of that associated with aging." The disease progresses from the epicardium toward the endocardium and involves at least the outer two thirds of the myocardium in about 75% of the cases.5
Moreover, the following 2 microscopic patterns have been described: (1) fatty or infiltrative and (2) fibrofatty or cardiomyopathic.3,14 These 2 forms differ with respect to location and pattern of adiposity, fibrosis, and myocarditis, as well as with regard to the likelihood of sudden arrhythmic death.
The fatty infiltrative type accounts for about 40% of the cases and has a high association with sudden death. Infundibular and apical aspects of the right ventricular free wall are most often involved. Affected regions are characterized by a lacelike pattern of adipose infiltration, progressing from epicardium toward the endocardium, and by interposed streams of normal or slightly atrophie myocytes. Fibrosis, wall thinning, and myocarditis, if present, tend to be mild. The left ventricle is usually spared.
In contrast, the fibrofatty cardiomyopathic type accounts for about 60% of the cases and has a high likelihood of congestive heart failure but a low likelihood of sudden death. The right ventricular free wall is usually diffusely involved, and affected areas show massive replacement of myocytes by fatty tissue, with residual subendocardial myocytes arranged in clusters and encircled by fibrous tissue. Right ventricular wall thinning and aneurysm formation are additional typical features. The left ventricle is also frequently involved by fatty infiltration, and the mitral valve may exhibit prolapse or a congenital cleft.
Focal infiltrates of T lymphocytes have been reported in 65% of the hearts in ARVCM,3-4 although Tabib et al6 identified myocarditis in only 5% of their 200 cases. Moreover, right ventricular inflammation affects the cardiomyopathic form of ARVCM substantially more often than the infiltrative form. dAmati et al14 reported myocarditis or borderline myocarditis in 44% of their cases with the cardiomyopathic form and in only 9% of those with the infiltrative form.
In the subendocardial region, right ventricular myocytes have been described as normal, atrophie, or hypertrophic. 2~4 Investigators have also described sarcoplasmic vacuolization and have attributed this to degeneration, mitochondriosis, or lipidosis.4,7 In the present case, large pale myocytes were evident in slides stained with Masson trichrome and resembled Purkinje cells. This new observation appears to differ from other reported sarcoplasmic changes.7 If such cells indeed have the electrophysiologic properties of Purkinje cells, they could contribute to the development of arrhythmias. In this regard, it is noteworthy that the present case represented the fibrofatty cardiomyopathic type of ARVCM, which otherwise tends to have a low likelihood of sudden arrhythmic death.
Association With Sudden Death
Annually in the United States, sudden unexpected death accounts for about half of all cardiovascular deaths, or approximately 350000 cases.15 The underlying causes vary with age. During the first 3 decades, myocarditis, cardiomyopathy, and coronary artery anomalies predominate as causes of sudden unexpected death. However, for persons older than 30 years, coronary atherosclerosis with ischemie heart disease is the most common cause. Although ARVCM is considered a rare disorder, it accounted for 10% of all cases of sudden unexpected cardiac death in the study by Tabib et al.6
Sudden death occurred during exertion in 30% of the 20 cases reported by Fornes et al5 and in 27% of the 13 cases described by Lobo et al.4 Furthermore, in the latter study, it was associated with acute emotional stress in 45% of cases. In the series of 200 cases by Tabib et al,6 sudden death occurred at home in 63%, on the street or at work in 13%, in the perioperative period in 10%, during sports activities in only 4%, and in other settings in 10%. Sadly, in most cases, sudden death is the first manifestation of ARVCM.5
In the present case, cardiac arrest occurred during a graduation ceremony. The diagnosis of ARVCM was first established at autopsy. Although historically it appeared to be nonfamilial, the children of the deceased may still be at risk for transmission of a gene defect that had manifested spontaneously in their father.
References
1. Cemaye! C, Pelliccia A, Thompson PD. Arrhythmogenic right ventricular cardiornyopathy. I Am Coll Cardiol. 2001 ;38:1 773-1 781.
2. Fontaine G, Fontaliran F, Herbert JL, et al. Arrhythmogenic right ventricular dysplasia. Annu Rev Med. 1999;50:17-35.
3. Thiene C, Basso C. Arrhythmogenic right ventricular cardiomyopathy: an update. Cardiovasc Pathol. 2001 ;10:109-11 7.
4. Lobo FV, Heggtveit HA, Butany J, Silver MD, Edwards JE. Right ventricular dysplasia: morphological findings in 13 cases. Can I Cardiol. 1992;8:261-268.
5. Forncs P, Ratel S, Lecomte D. Pathology of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an autopsy study of 20 forensic cases. J Forensic Sd. 1998;43:777-783.
6. Tabib A, Loire R, Chalabreysse L, et al. Circumstances of death and gross and microscopic observations in a series of 200 cases of sudden death associated with arrhythmogenic right ventricular cardiomyopathy and/or dysplasia. Circulation. 2003;108:3000-3005.
7. d'Amati G, di Gioia CRT, Giordano C, GaIIo P. Myocyte transdifferentiation: a possible pathogenetic mechanism for arrhythmogenic right ventricular cardiomyopathy. Arch Pathol Lab Med. 2000;124:287-290.
8. Nagata M, Hiroe M, lshiyama S, et al. Apoptotic cell death in arrhythmogenic right ventricular cardiomyopathy. Jpn Heart]. 2000;41:733-741.
9. Hamid MS, Norman M, Quraishi A, et al. Prospective evaluation of relatives for familial arrhythmogenic right ventricular cardiomyopathy/dysplasia reveals a need to broaden diagnostic criteria. J Am Coll Cardiol. 2002;40:1445-1450.
10. Fatkin D, Graham RM. Molecular mechanisms of inherited cardiomyopathies. Physio/ Rev. 2002;82:945-980.
11. Towbin JA. Molecular genetic basis of sudden cardiac death. Cardiovasc Pathol. 2001:10:283-295.
12. Alcalai R, Metzger S, Rosenheck S, Meiner V, Chajek-Shaul T. A recessive mutation in desmoplakin causes arrhythmogenic right ventricular dysplasia, skin disorder, and woolly hair. I Am Coll Cardiol. 2003;42:319-327.
13. Burke AP, Farb A, Tashko G, Virmani R. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: are they different diseases? Circulation. 1998:97:1571-1580.
14. d'Amati G, Leone O, di Gioia CRT, et al. Arrhythmogenic right ventricular cardiomyopathy: clinicopathologic correlation based on a revised definition of pathologic patterns. Hum Pathol. 2001:32:1078-1086.
15. Burke AP, Farb A, Virmani R. Sports-related and non-sports-related sudden death in young adults. Am Heart I. 1991;121:568-575.
Dongjiu Ye, MD, PhD; William D. Edwards, MD; Waheeb Rizkalla, MD
Accepted for publication June 15, 2005.
From the Department of Pathology, Conemaugh Memorial Medical Center, Johnstown, Pa (Drs Ye and Rizkalla); and Department of Laboratory Medicine and Pathology, Mayo Clinic, and Department of Pathology, Mayo College of Medicine, Rochester, Minn (Dr Edwards).
The authors have no relevant financial interest in the products or companies described in this article.
Reprints: Dongjiu Ye, MD, PhD, Department of Pathology, Conemaugh Memorial Medical Center, 1086 Franklin St, Johnstown, PA 15905-4398 (e-mail: dye@conemaugh.org).
Copyright College of American Pathologists Oct 2005
Provided by ProQuest Information and Learning Company. All rights Reserved