Members of a family have been investigated because of three sudden deaths among them. Two young sisters, aged 12 and 16, died suddenly while swimming and running, while their 19-year-old brother died suddenly during emotional stress. In no case did autopsies reveal any structural abnormalities. Their 39-year-old mother and her 19-year-old daughter gave a history of syncopes, while having a normal physical examination and normal ECGs. During a treadmill test, multiple ventricular extrasystoles and bursts of polymorphic ventricular tachycardia were provoked. Patient-members of this family have undergone echocardiography, catheterization of the left and right sides of the heart, endomyocardial biopsy, and electrophysiologic studies. A differential diagnosis of an inherited long QT interval syndrome, catecholamine-induced arrhythmias, and arrhythmogenic right ventricular dysplasia have been suggested. Patients were given atenolol and were followed up for 18 months. This therapy has greatly reduced the exertional arrhythmias as assessed by serial treadmill tests.
(CHEST 1997; 111:1130-33)
Key words: beta blockers, exertional ventricular arrhythmias; polymorphic ventricular tachycardia; sudden cardiac death; treadmill test
Ventricular arrhythmias can occur in a structurally normal heart. Such syndromes presenting different clinical and electrocardiographic features already have been described. One syndrome refers to patients with a prolonged QT interval with or without deafness.[1,2] These individuals present with ventricular arrhythmias and torsades de pointes ventricular tachycardia related to adrenergic stimulation and may sustain attacks of syncope and sudden cardiac death.[1,2] The syndrome of arrhythmogenic right ventricular dysplasia is clinically characterized by palpitations, ventricular arrhythmias, and sudden death during exertion.[3,4] Coumel and colleagues[5,6] described a group of children who presented with syncope related to exercise or to emotion; they had normal ECGs at rest, and polymorphic ventricular tachycardia could be induced with exercise. This has been named as the syndrome of catecholamine-induced ventricular arrhythmias. Brugada and Brugada[7] reported on a rare syndrome characterized by right bundle branch block, persistent ST segment elevation in right precordial leads, and sudden cardiac death.
The diseases, however, responsible for sudden cardiac death in young individuals are myriad.[8] The most common cardiac disease causing sudden death in young people is hypertrophic cardiomyopathy.[9-11] Valvular aortic stenosis, myocarditis, congenital anomalies of the coronary arteries, and Marfan's syndrome have been reported as some other causes for sudden cardiac death.[10,11] In still other cases, however, the cause of sudden death is never found.
In this article, we report family members who present with syncope, exertional ventricular arrhythmias, polymorphic ventricular tachycardia, and sudden deaths related to physical exercise or emotional stress.
CASE REPORTS
CASE 1
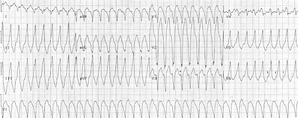
A 19-year-old woman (Fig. 1, III-18) was referred to us for cardiology evaluation in April 1994. She reported an episode of syncope Her physical examination revealed no gross abnormalities. Her standard 12-lead ECG showed sinus bradycardia of 47 beats per minute (bpm), a normal P wave axis, a P wave duration, and amplitude in standard lead II at 100 ms and 160 [micro]V, respectively. The PR interval in standard lead II was 148 ms; QRS and T wave axes were normal. The QT interval in lead [V.sub.2] measured 404 ms, and the QT interval, corrected for heart rate, was 367 ms. There were prominent positive U waves in leads [V.sub.2], [V.sub.3], and [V.sub.4]. A chest x-ray film suggested mild right ventricular dilatation, and two-dimensional echocardiogram, and Doppler study of the heart revealed no abnormality. Results of hematologic, biochemistry, renal, hepatic, thyroid, and pheochromocytoma tests were normal. Findings from MRI studies of the heart were compatible with adipose infiltration in the right ventricular area. The patient underwent a stress test on a motorized treadmill (Quinton 5000; Quinton Instruments Co; Bothell, Wash) with the standard Bruce protocol; at the second minute of exercise at a heart rate of 110 bpm ventricular extrasystoles were triggered. Extrasystoles had a normal axis with a left bundle branch block pattern, while others had a left axis with a concordant Rs configuration throughout the precordial leads. At a heart rate of 134 bpm, short bursts of polymorphic ventricular tachycardia were detected, and, therefore, the test was discontinued (Fig 2). At a heart rate of 57 bpm, arrhythmias were abolished by the third minute in recovery. Propranolol hydrochloride, 10 mg tid, a regimen that has proved to be ineffective as shown, with poor results on repeated treadmill tests. We proceeded with catheterization of the left and right sides of the heart; results of coronary arteriography were normal, and the diastolic pressures in right atrium, right ventricle, and left ventricle were 10, 10, and 15 mm Hg, respectively, with a deep-plateau form. During electrophysiologic studies, there were no baseline long QT or QTU intervals, and no U waves developed when isoproterenol hydrochloride infusion was started, despite provocation of ventricular extrasystoles. Ventricular stimulation was not performed. Myocardial biopsy showed hypertrophy of myocytes without other signs of myopathy.
[Figure 1III-18,2 ILLUSTRATION OMITTED]
CASE 2
The 39-year-old mother of patient 1 (Fig. 1, II-10) had a cardiac evaluation in April 1994. She had six children. Two of her daughters (Fig 1, III-20 and III-21) died suddenly; one who was 12 years old died during a swimming competition and the other who was 16 years old died while running in a competition. Her 19-year-old son (Fig 1, III-19) also died suddenly in July 1994 immediately after his involvement in a minor car accident; he was driving a stolen car without a driver's license. His death was not related to trauma. The mother (Fig 1, II-10), her son (Fig 1, III-19) who died in the car accident, and his twin sister (Fig 1, III-18) who is alive, were diagnosed with ventricular arrhythmias and polymorphic ventricular tachycardia related to exercise.
[Figures 1II-10, III-18,19,20,21 ILLUSTRATION OMITTED]
Patient 2 (the mother of patient 1) described an incident of fainting while on a long walk. Her father (Fig 1, I-4) and mother (Fig 1, I-3), 65 and 73 years old, respectively, are alive and free of any diagnosed cardiac disease.
[Figures 1I-3-4 ILLUSTRATION OMITTED]
CASE 3
Results of a physical examination on the husband of patient 2 were normal. A 12-lead ECG showed a sinus rhythm, a normal P wave axis, a P wave duration and amplitude in the standard lead II of 90 ms and 110 [micro]V, respectively, a PR interval in the standard lead II of 145 ms, and normal QRS and T wave axes. The QRS duration measured 90 ms, the QT interval was 405 ms long in lead [V.sub.2], and the QT corrected for heart rate with the Bazett formula was 380 ms. A chest x-ray film showed a normal cardiovascular silhouette, while results of biochemistry, hematologic, renal, hepatic, thyroid, and pheochromocytoma investigations were normal. A two-dimensional echocardiogram and Doppler echocardiography failed to reveal any structural cardiac abnormality.
This patient was exercised with the standard Bruce protocol on a motorized treadmill. After 3.56 min of exercise, at a heart rate of 133 bpm, ventricular extrasystolic beats at the end of the T wave were triggered. Extrasystoles had a concordant RS pattern throughout the precordial leads and an inferior QRS axis, while other extrasystoles had a normal axis and a left bundle branch block pattern. As exercise progressed, at a heart rate of 139 bpm, ventricular couplets and short bursts of nonsustained polymorphic ventricular tachycardia appeared (Fig 3). Exercise was terminated immediately. There were no arrhythmias observed after the first minute of recovery at a heart rate of 136 bpm. The patient was treated with propranolol, 10 mg kid. This therapy, however, did not abolish arrhythmias while the patient was exercising, as demonstrated by repeated treadmill tests. We proceeded with catheterization of the left and right sides of the heart; left ventricular function, as assessed with contrast ventriculography, coronary arteriography, left ventricular and aortic pressures, catheterization of the right side of the heart, and pressures of the right side of the heart were normal. During endomyocardial biopsy, an accidental right ventricular perforation occurred. Emergency pericardiocentesis and evacuation of 200 mL of blood from the pericardial cavity were performed. During an electrophysiologic study, there were no baseline long QT or QTU intervals, and no U waves developed upon isoproterenol infusion (despite appearance of ventricular extrasystoles). Programmed right ventricular stimulation (up to 3 extrastimuli at basic cycle lengths of 600 and 400 ms from 2 sites) did not provoke any significant arrhythmia. The cardiac biopsy specimen was normal too.
[Figure 3 ILLUSTRATION OMITTED]
FAMILY STUDY
In view of the medical histories and clinical findings of our patients, a family study was undertaken. The physicians of the other two children of patient 2 who are alive, the 15-year-old (Fig 1, III-22) and the 13-year-old (Fig 1, III-23), reported that both children were free of symptoms and had normal ECGs, chest-x-ray films, and stress tests. We recruited C.G., the father of patient 2 (Fig 1, I-4), who is 65 years old, and A.G., her mother (Fig 1, I-3), who is 73 years old. Both were asymptomatic and had normal 12-lead ECGs and chest x-ray films. The father exercised for 6.35 min with the Bruce protocol, achieving a heart rate of 153 bpm corresponding to 95% of his maximal predicted heart rate. He remained symptom-free throughout exercise; his blood pressure response was normal and no arrhymias were provoked with exercise. The mother exercised for 3.20 min, achieving a heart rate of 143 bpm corresponding to 100% of her maximal predicted heart rate. No arrhymias were provoked with exercise. The other four sisters of the husband of patient 2 (Fig 1, II-3, II-4, II-6, II-8) also were recruited and results of their examinations were normal; ECGs and treadmill tests also were normal. Patient 3 (husband of patient 2 [Fig 1, II-11]) is alive; however, he is not willing to be examined. He divorced patient 2, married again, and had two children (Fig 1; III-24 and III-25) with his second wife (Fig 1, III-12).
[Figures 1-II-4,6,8,11, III-12,22,23,24,25 ILLUSTRATION OMITTED]
Patient 2 and her daughter, patient 1 (Fig 1, II-10 and III-18) were followed up for 18 months. They were treated with atenolol, 150 and 200 mg daily, respectively, and have undergone serial treadmill tests. The first one (patient 2) can exercise for 10 min with the Bruce protocol without provocation of any arrhythmias; her heart rate never exceeded 105 bpm with exercise. The second (patient 1) can exercise for 6 min with the Bruce protocol; ventricular extrasystolic beats usually are provoked with exercise but without ventricular tachycardia.
DISCUSSION
The sudden death of a young person is always a tragic and devastating event. The most common cardiac disorder reported to be responsible for such a catastrophe is hypertrophic cardiomyopathy.[8-10] Other structural cardiac disorders responsible for sudden deaths are aortic stenosis, Marfan's syndrome, right ventricular cardiomyopathy, or anomalous origin of a coronary artery.[8-11] Cardiac diseases characterized by electrophysiologic abnormalities, such as long QT interval syndromes or catecholamine-induced ventricular arrhythmia syndromes, are reported to be causes for sudden cardiac deaths as well.[1,2,5,6]
In this article, we describe a syndrome characterized by exercise-induced ventricular arrhythmias, recurrent episodes of syncope, and exercise-related sudden deaths occurring in a family. The disease in this family is expressed as an autosomal dominant trait in the absence of any structural heart abnormality. The 12-year-old daughter died while swimming with other children in front of a crowd in an official school competition. The 16-year-old daughter died during a competition while running with other children to win a prize. The 19-year-old son, who had no driving license, had an accident while driving a stolen car. He was not injured, but appeared to be emotionally disturbed, as corroborated by witnesses, and died suddenly a few minutes later. In no case did autopsies reveal structural cardiac or extracardiac abnormalities.
In this rare case, differential diagnosis mainly involves syndromes of catecholamine-induced ventricular arrhythmias, long QT interval syndromes, and arrhythmogenic right ventricular dysplasia.
Though there are no strict criteria for defining normal and abnormal QT interval, QT intervals in our patients are no longer than 400 ms in serial ECGs. These values are not diagnostic of a long QT interval and are quite shorter than those of 600 ms, which are suggested to distinguish patients at risk for torsades de pointes and sudden death.[1] The question of whether U waves -should be included in the measurements has not been answered yet.[1] However, there are reported cases with a normal QT interval, familial syncope, and sudden death that have been included in such syndromes.[1] As described by Shimizu and his colleagues,[1,2] people with documented long QT interval demonstrated QT prolongation with exercise and with isoproterenol administration. In our patients, QT interval shortens with exercise as shown on stress electrocardiographic tracings, and no QT prolongation is provoked with isoproterenol infusion during electrophysiologic study.
Though the MRI suggested adipose infiltration in the right ventricular area[13] in patient 1 (Fig 1, III-18), autopsies in the other patients who died suddenly did not reveal any structural cardiac abnormalities. Studies have shown that right ventricular cardiomyopathy can be detected with postmortem examination.[4] In addition, histologic studies did not reveal adipose of fibrous infiltration in the right ventricular region, findings suggesting such a diagnosis.[4] On the other hand, elevated pressures in the right atrium and ventricle, the deep-plateau pressure configuration in the right ventricle, and the otherwise accidental perforation of the right ventricle in one of our patients during endomyocardial biopsy may be suggestive of some kind of right ventricular cardiomyopathy or dysplasia.[3,13]
The fact that the mother and four out of the six children of this family are affected probably implies a familial character of this disorder. The disease seems to be inherited in an autosomal dominant fashion. However, other close relatives (aunts and cousins) seem to be spared by the disease. The observed genetic anticipation may be explained by a new mutation (base-pair substitution, deletion, insertion, or an unstable trinucleotide repeat, or all of these).
Deaths occurred during strenuous exercise or during emotional distress, situations in which there is an excessive catecholamine release. Ventricular arrhythmias and polymorphic ventricular tachycardia are well documented with several treadmill tests, always in the absence of a long QT interval. Therefore, we believe that these characteristics favor the diagnosis of an inherited syndrome with catecholamine-induced ventricular arrhythmias.
ACKNOWLEDGMENTS: We would like to thank Mrs. Elena Kyriacou for her typing and editorial assistance and Mrs. Ellie Papaphilippou for her editorial assistance.
REFERENCES
[1] Jackman W, Friday K, Anderson J, et al.The long QT syndromes: a critical review, new clinical observations and a unifying hypothesis. Progr Cardiovasc Dis 1988; 2:115-72
[2] Swartz PJ. Idiopathic long QT syndrome: progress and questions. Am Heart J 1985; 109:399-411
[3] Marcus F, Fontaine G, Guiraudon G, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation 1982; 65:384-98
[4] Thiene G, Nava A, Corrado D, et al. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med 1988; 318:129-33
[5] Coumel P, Fidelle J, Lucet V, et al. Catecholamine-induced severe ventricular arrhythmias with Adams-Stokes syndrome in children: report of four cases. Br Heart J 1978; 40(suppl):28-37
[6] Coumel P, Leenhardt A, Jaddad G. Exercise ECG: prognostic implications of exercise induced arrhythmias. Pace 1994; 117:417-27
[7] Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. J Am Coll Cardiol 1992; 20:1391-96
[8] Braunwald E. Heart disease: a textbook of cardiovascular medicine (vol 1). 3rd ed. Philadelphia: WB Saunders, 1988; 746-47
[9] Maron BJ, Boberts WC, Epstein SE. Sudden death in hypertrophic cardiomyopathy: a profile of 78 patients. Circulation 1982; 65:1388-94
[10] Lambert E, Menon V, Wagner H, et al. Sudden unexpected death from cardiovascular disease in children: a cooperative international study. Am J Cardiol 1974; 34:89-96
[11] Maron B, Epstein S, Roberts W. Causes of sudden death in competitive athletes. J Am Coll Cardiol 1986; 7:204-14
[12] Shimizu W, Ohe T, Kurita T, et al. Differential response of QTU interval to exercise, isoproterenol, and atrial pacing in patients with congenital long QT syndrome. Pace 1991; 14:1966-70
[13] Ricci C, Congo R, Pagnan L, et al. Magnetic resonance imaging in right ventricular dysplasia. Am J Cardiol 1992; 70:1589-95
(*) From the Department of Cardiology, Nicosia General Hospital (Drs. Myrianthefs, Minas, and Zambartas) and the Cyprus Institute of Neurology and Genetics (Dr. Cariolou), Nicosia, Cyprus; and the Cardiology Department, Chaim Sheba Medical Center, Tel Hashomer Hospital, Tel Hashomer, Israel (Dr. Eldar). Manuscript received June 12, 1996; revision accepted September 11. Reprint requests: Dr. Myrianthefs, 18, Vassilissis Friderikis Str, Ayios Dhometios 2360, Nicosia, Cyprus
COPYRIGHT 1997 American College of Chest Physicians
COPYRIGHT 2004 Gale Group