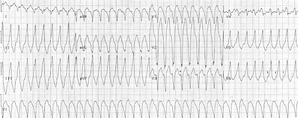
A 10-year-old boy presented with progressive weakness and lethargy. Prior to this episode, he was generally healthy and active. he was born 6 weeks premature and had a history of chronic sinus problems and periodic eczema and psoriasis. In early childhood, he had a diagnosis of immunoglobulin A and G (IgA and IgG) deficiency, which apparently resolved. His only medications were topical medications for eczema and psoriasis.
The onset of his lethargy had coincided with an upper respiratory infection 3 weeks prior to his presentation and had advanced to the point that he was sleeping almost constantly. he was hospitalized after he developed increased difficulty breathing, progressive edema, and poor oral intake. A physical examination showed stable vital signs but severe cardiomegaly and congestive heart failure. Chest x-ray films showed an enlarged heart, and an electrocardiogram showed episodes of ventricular tachycardia. During his hospitalization, his condition stabilized, and the edema associated with congestive heart failure resolved. A diagnosis of viral cardiomyopathy associated with prior upper respiratory tract infection was rendered. The boy continued to be symptomatic and to have dilated, hypocontractile ventricles. Electrocardiography showed poor function of the right and left ventricles with an atrialventricular valve regurgitation that was more pronounced on the right. Electrocardiograms showed a normal sinus rhythm with rare premature beats and T-wave abnormalities that were unchanged from those during his initial hospitalization. he was relatively stable, with profound fatigue, but he showed neither improvement nor progression of his heart disease during the next 8 months, at which time a cardiac transplantation was performed.
The explanted heart was markedly enlarged with a distended, flaccid right ventricular wall. The right ventricular wall was thin (1-3 mm) and partially translucent; the endocardial surface was atypically trabeculated with prominent pectinate muscles (Figure, A). The left ventricular wall was thicker (9-11 mm), and the myocardium was "marbled," with small pale-white areas beneath the epicardium and a thin rim of epicardial fat superimposed on muscle (Figure, B).
Microscopically, large portions of the right ventricular myocardium were replaced by adipose tissue (Figure, C; trichrome stain; a, adipose tissue; 1, ventricular lumen). Remaining cardiac myocytes were hypertrophie with a bizarre nuclear morphology (data not shown). Bands of fibrous and adipose tissue separated the cardiac myocytes. Similar changes were present in the left ventricle, but fibrosis predominated over fatty replacement, and the myocardium was better preserved (Figure, D; trichrome stain; a, adipose tissue).
What is your diagnosis?
Pathologie Diagnosis: Arrhythmogenic Right Ventricular Cardiomyopathy, Cardiomyopathic Pattern
This patient presented clinically with a dilated cardiomyopathy that was originally thought to be secondary to viral myocarditis. However, the pathology of the explanted heart was consistent with arrhythmogenic right ventricular cardiomyopathy (ARVC) of the cardiomyopathic subtype.
The clinical diagnosis of ARVC, also termed arrhythmogenic right ventricular dysplasia, is difficult. Many patients are asymptomatic and are found to have ARVC only at autopsy following a sudden, unexpected death. In the United States, it is estimated that ARVC accounts for 5% of all sudden cardiac deaths among people younger than 65 years. In the Veneto region of Italy, the condition is reported to be the most common cause of sudden arrhythmic deaths in people younger than 35 years.1 Patients who are symptomatic present with a variety of clinical signs, including right ventricular structural changes, electrocardiographic alterations, and arrhythmias. Structural changes in ARVC include a severe dilation of the right ventricle, right ventricular aneurysms, and right ventricular regional hypokinesia. Impairment of right ventricular function with a decreased ejection fraction is accompanied by little or no dysfunction of the left ventricle. Electrocardiographic changes include prolongation of the QRS complex and inverted T waves in the right precordial leads. Arrhythmias that may be present include a left bundle branch block, tachycardia, and frequent ventricular extrasystole.1 Evaluation of the right ventricle by angiocardiography, echocardiography, or magnetic resonance imaging may help in the diagnosis of ARVC.2 Angiocardiography shows akinetic or dyskinetic "bulges" of the right ventricle. Echocardiography is an effective tool in evaluating patients with suspected ARVC because it evaluates right ventricular wall thickness and end diastolic volume and function. Magnetic resonance imaging is helpful because it differentiates adipose tissue from muscle, although motion artifacts can obscure the image quality and make interpretation difficult.1
The primary pathologic feature of ARVC is the replacement of myocardium with fibrofatty tissue. This infiltration progresses from the subepicardium to the endocardium, leaving scattered islands of residual myocytes and a subendocardial rim of intact myocardium. The remaining cardiac myocytes are hypertrophie with bizarre nuclear features.1 A "triangle of dysplasia" is often most affected, which consists of the diaphragmatic, apical, and infundibular regions of the right ventricle. In 50% of the cases at autopsy, aneurysmal dilations are found in the wall of this triangle.2
Other conditions that must be considered in a pediatrie patient with dilated cardiomyopathy include infection (eg, viral myocarditis, toxoplasmosis), toxins (eg, anthracyclines), ischemie injury, autoimmune disease, muscular dystrophy, and a variety of metabolic disorders. Interstitial fibrosis with or without inflammation is the unifying feature of most of these conditions. Abundant fatty infiltration, lack of inflammation, and clinical data (eg, history of anthracycline exposure) or laboratory data (eg, serologie evidence of an autoimmune disease) differentiate ARVC from most of these other forms of dilated cardiomyopathy. However, dilated cardiomyopathy often remains idiopathic, despite thorough clinical and pathologic evaluations.
A diagnosis of ARVC by myocardial biopsy is problematic. The diagnostic changes often lie deeper in the myocardium than can usually be sampled in the myocardial biopsy.1 Several other processes can also cause fibrofatty infiltration of the myocardium. ARVC can be differentiated from other entities that cause fibrofatty infiltration of the myocardium by the presence of more than 3% fibrous tissue and more than 40% fatty tissue on biopsy sections.1,2
Two distinct subtypes of ARVC have been described. The infiltrative or fatty form affects the right ventricle primarily, usually in the apical and infundibular regions, and more commonly presents as sudden death.3 The right ventricular wall thickness is frequently within the reference range.2 The cardiomyopathic form of ARVC usually has biventricular involvement. The right ventricular free wall is thinned (
The cause of ARVC is unknown, but multiple theories have been advanced, evoking degenerative or infectious processes and genetic disorders. Linkage studies have associated ARVC with loci at Iq42-43, 14q23-24, 14q42-43, 2q32, 3q23, and 17q21.4 A defect in 14q23-24 may affect the [alpha]-actin gene, which encodes a cytoskeletal protein analogous to dystrophin. It has been speculated that ARVC is a "myocardial dystrophy" with genetically determined muscular atrophy and secondary replacement by adipocytes similar to Duchenne muscular dystrophy.1 Alternatively, a transdifferentiation theory proposes that the adipocytes in ARVC arise from cardiac myocytes through the progressive replacement of myofibrils by fat droplets. A transitional zone between cardiac muscle and adipose tissue, which expressed both desmin and vimentin, was demonstrated in one patient.5 The finding of inflammatory infiltrates within ARVC hearts has led to the theory that myocarditis initiates the destruction of cardiac myocytes and their subsequent replacement with adipocytes.1 Finally, high levels of a cysteine protease required for apoptosis in 6 of 8 patients with ARVC led to an apoptotic theory of cardiac myocyte death with the subsequent replacement by adipocytes.1
References
1. Gamayel C, Relliccia A, Thompson PD. Arrhythmogenic right ventricular cardiomyopathy. I Am Coll Cardiol. 2001 ;38:1 773-1 781.
2. Corrado D, Basso C, Thiene G. Arrhythmogenic right ventricular cardiomyopathy: diagnosis, prognosis and treatment. Heart. 2000:83:588-595.
3. d'Amati G, Leone O, di Gioia CRT, et al. Arrhythmogenic right ventricular cardiomyopathy: dincopathological correlation based on a revised definition of pathological patterns. Hum Pathol. 2001;32:1078-1086.
4. Bauce B, Nava A, Rampazzo A, et al. Familial effort polymorphic ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy map to chromosome 1q42-43. Am I Cardiol. 2000;85:573-579.
5. d'Amati G, di Gioia CRT, Giordano C, GaIIo P. Myocyte transdifferentiation: a possible pathogenic mechanism for arrhythmogenic right ventricular cardiomyopathy. Arch Pathol Lab Med. 2000;124:287-290.
Sandra White, MSIII; Joseph R. Siebert, PhD; Raj P. Kapur, MD, PhD
Accepted for publication February 3, 2004.
From the Loma Linda School of Medicine, Loma Linda, Calif (Ms White); and the Department of Pathology, Children's Hospital and Regional Medical Center, University of Washington, Seattle (Drs Siebert and Kapur).
The authors have no relevant financial interest in the products or companies described in this article.
Corresponding author: Raj P. Kapur, MD, PhD, Department of Pathology, 6P-1, Children's Hospital and Regional Medical Center, 4800 Sand Point Way NE, Seattle, WA 98105 (e-mail: raj.kapur@ seattlechildrens.org).
Reprints not available from the authors.
Copyright College of American Pathologists Jun 2004
Provided by ProQuest Information and Learning Company. All rights Reserved