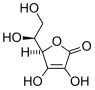
Ascorbic acid
Ascorbic acid is an organic acid with antioxidant properties. Its appearance is white to light yellow crystals or powder. It is water soluble. The L-enantiomer of ascorbic acid is commonly known as vitamin C. In 1937 the Nobel Prize for chemistry was awarded to Walter Haworth for his work in determining the structure of ascorbic acid (shared with Paul Karrer, who received his award for work on vitamins), and the prize for Physiology or medicine that year went to Albert Szent-Györgyi for his studies of the biological functions of L-ascorbic acid. more...
Chemistry
Acidity
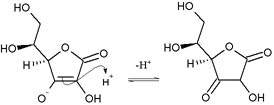
The hydroxyls (OH) next to the bottom double bond are enols. One enol loses an electron pair, becoming an oxonium group (=OH+), by creating a double bond to the carbon. Simultaneously, the carbon-carbon double bond (between the enols) transfers its electrons to form a double bond to the next (two-oxygen) carbon. To give way, the double bond electrons of the carbonyl are received by the carbonyl's oxygen, to produce an enolate. The oxonium promptly deprotonates to produce a carbonyl, and this loss of protons gives ascorbic acid its acidity. The overall reaction is enol deprotonation to produce an enolate, where the negative charge of the resulting enolate counterion is delocalized over the system of carbonyl (C=O) and the double bond (C=C). This delocalization makes the counterion more stable and less likely to regain the proton.
Tautomerism
Ascorbic acid also rapidly interconverts into two unstable diketone tautomers by proton transfer, although it is the most stable in the enol form. The proton of the enol is lost, and reacquired by electrons from the double bond, to produce a diketone. This is an enol reaction. There are two possible forms, 1,2-diketone and 1,3-diketone.
Uses
Ascorbic acid is easily oxidized and so is used as a reductant in photographic developer solutions (among others) and as a preservative.
Exposure to oxygen, metals, light and heat destroy ascorbic acid, so it must be stored in dark and cold and not in a metal containment.
The oxidized form of ascorbic acid is known as dehydroascorbic acid.
The L-enantiomer of ascorbic acid is also known as vitamin C (the name "ascorbic" comes from its property of preventing and curing scurvy). Primates (including humans) and a few other species in all divisions of the animal kingdom, notably the guinea pig, have lost the ability to synthesise vitamin C and must obtain it in their food.
Ascorbic acid and its sodium, potassium, and calcium salts are commonly used as antioxidant food additives. These compounds are water soluble and thus cannot protect fats from oxidation: for this purpose, the fat-soluble esters of ascorbic acid with long-chain fatty acids (ascorbyl palmitate or ascorbyl stearate) can be used as food antioxidants.
The relevant European food additive E numbers are: E300 ascorbic acid, E301 sodium ascorbate, E302 calcium ascorbate, E303 potassium ascorbate, E304 fatty acid esters of ascorbic acid (i) ascorbyl palmitate (ii) ascorbyl stearate.
Antioxidant mechanism
Ascorbate acts as an antioxidant by being itself available for energeticaly favourable oxidation. Oxidants (scientifically referred to as reactive oxygen species) such as the hydroxyl radical (formed from hydrogen peroxide), contain an unpaired electron and thus are highly reactive and damaging to humans and plants at the molecular level. This is due to their interaction with nucleic acid, proteins and lipids. Reactive oxygen species can 'abstract' a hydrogen from ascorbate, which becomes monodehydroascorbate and soon gains another electron to become dehydroascorbate. The reactive oxygen species are reduced to water while the oxidized forms of ascorbate are relatively stable and unreactive, and do not cause cellular damage.
Read more at Wikipedia.org