Blood culture--negative endocarditis is common in Algeria. We describe the etiology of infective endocarditis in this country. Samples from 110 cases in 108 patients were collected in Algiers. Blood cultures were performed in Algeria. Serologic and molecular analysis of valves was performed in France. Infective endocarditis was classified as definite in 77 cases and possible in 33. Causative agents were detected by blood cultures in 48 cases. All 62 blood culture-negative endocarditis cases were tested by serologic or molecular methods or both. Of these, 34 tested negative and 28 had an etiologic agent identified. A total of 18 infective endocarditis cases were caused by zoonotic and arthropodborne bacteria, including Bartonella quintana (14 cases), Brucella melitensis (2 cases), and Coxiella burnetii (2 cases). Our data underline the high prevalence of infective endocarditis caused by Bartonella quintana in northern Africa and the role of serologic and molecular tools for the diagnosis of blood culture--negative endocarditis.
**********
In Algeria, infective endocarditis is common. Vegetations graft primarily on lesions of rheumatic heart disease (1,2). The rate of blood culture-negative endocarditis in Algeria is as high as 76% (2), which leads to difficulty in antimicrobial treatment. Most cases of blood culture--negative endocarditis have been thought to be caused by previous antimicrobial therapy. Infective endocarditis prognosis is often obscured by delayed diagnosis and a lack of specific treatment. In Algeria, poor socioeconomic level and lack of medical follow-up of patients are among the factors associated with endocarditis. The concentration of medical infrastructures in the northern part of the country leads to the referral of patients with serious illnesses, such as endocarditis, to northern hospitals, especially within Algiers (Figure 1). Algiers, the capital and largest city with [approximately equal to] 5 million inhabitants, has 7 hospitals, including 6 cardiology and 5 cardiac surgery wards. These wards receive patients with endocarditis, either for diagnosis and treatment or for corrective surgery of postendocarditis lesions. A retrospective analysis of Algerian infective endocarditis cases showed streptococci and staphylococci were the leading causes, followed by less frequent causes, such as enterobacteria and Haemophilus spp. (2). A high percentage of blood culture--negative endocarditis was noted. However, no study has evaluated the agents responsible for blood culture--negative endocarditis. New serologic and molecular tools, which have improved the etiologic diagnosis of infective endocarditis, have not been used to clarify the unknown role of fastidious bacteria (3-11). In our study, samples were collected from 110 patients with suspected cases of endocarditis. All samples were analyzed prospectively by using conventional microbiologic methods in Algiers. When available, cardiac valves and serum samples were stored to perform retrospective analysis at the Unite des Rickettsies (Marseille, France).
[FIGURE 1 OMITTED]
Material and Methods
Patients
Clinicians usually diagnose infective endocarditis by using the modified Duke criteria, which includes 3 major criteria (blood cultures typical of infective endocarditis, vegetations on echocardiography, and Coxiella burnetii serologic testing with immunoglobulin [Ig] G phase 1 titer [greater than or equal to] 1:800) and 7 minor criteria (positive blood cultures, fever, previous heart disease, arterial embolism, positive results on serologic examination for endocarditis bacterial pathogens, immunologic disorders, and atypical but compatible findings on echocardiography) (12). Definite infective endocarditis is diagnosed if any of the following conditions is met: 2 major criteria exist; 1 major criterion and 3 minor criteria; or 5 minor criteria. Possible infective endocarditis is considered if 1 major criterion and 1 minor criterion or 3 minor criteria exist. On the basis of these criteria, we could locate 110 cases in 108 patients with definite or possible infective endocarditis in 5 cardiology wards and 2 cardiac surgery wards in Algiers during a 42-month period (June 2000-December 2003). For each patient, an information sheet with epidemiologic, clinical, echocardiographic, and biologic data was filled out. A minimum of 3 blood cultures were sampled per patient. Thirty-eight cardiac valve specimens from 38 (35.4%) patients were sampled and stored at -80[degrees]C. Thirty-seven cardiac valve specimens from another 30 (27.3%) patients were formalin-fixed for pathologic testing. Sixty-one serum samples from 61 (55.5%) patients were available.
Blood Cultures
Either Castaneda Aer/Anaer (Bio-Rad, Marnes-La-Coquette, France) or broth for blood culture (Institut Pasteur d'Algerie, Algiers, Algeria) were used as blood-culture medium and were incubated at 37[degrees]C. If signs of culture appeared, a blood sample was taken from the culture bottle and Gram staining on Columbia blood agar (BioMerieux, Marcy L'Etoile, France) and chocolate agar (BioMerieux) was performed. Agar plates were incubated in 5% C[O.sub.2] at 37[degrees]C. In the event of culture, the microorganism was identified by API identification tests (BioMerieux). At day 15 of incubation, if cultures remained negative, an enrichment of each bottle was processed on Todd-Hewitt broth (Institut Pasteur d'Algerie) supplemented with 0.01% L-cysteine (Sigma-Aldrich, Lausanne, Switzerland) and 0.001% hypochloride pyridoxal (Sigma-Aldrich). In cases of broth turbidity, microscopic examinations were performed as described above. If culture was positive, the strain was identified.
Valve Analysis
Axenic Culture
Thirty-eight excised cardiac valves were examined. If macroscopic lesions of infective endocarditis were detected, we attempted to divide the valve into 3 parts to be used for bacteriologic analysis, storage at -80[degrees]C, and histologic analysis. Portions of valve tissue were ground with a mortar and pestle and cultured on Columbia blood agar and chocolate agar supplemented with Polyvitaminic Supplement (Bio-Rad) at 35[degrees]C for 15 days in 5% C[O.sub.2]. We performed direct Gram staining and identified colonies as described above.
Cell Culture
Cell cultures were performed in France. Specimens from 12 cardiac valves positive on polymerase chain reaction (PCR) for Bartonella quintana or Brucella melitensis were spread onto cells grown within a shell vial as previously described (13,14). After 3 weeks of incubation at 37[degrees]C, the bacteria were detected by using Gimenez staining, a direct immunofluorescence test incorporating polyclonal antibodies directed against Bartonella, and by PCR targeting the 16S rRNA sequence.
Molecular Biology
For the 38 cardiac samples stored at -80[degrees]C, molecular analysis was performed in France. After 18 hours of proteinase K digestion at 55[degrees]C, DNA was extracted from tissue by using the MagNA Pure LC instrument (Roche Molecular Biochemicals, Manheim, Germany) and MagNA Pure LC DNA Isolation Kit III (Roche Molecular Biochemicals), as described by the manufacturer. A PCR-positive valve sample taken from a patient with Staphylococcus aureus endocarditis was used as a positive control. A mixture of all reagents used for DNA extraction and DNA extracted from normal heart tissue were processed as negative controls. One negative control was included for every 5 samples tested. PCR amplification and sequencing were performed, as previously described (15), by using primers in Table 1. PCR targeting the 16S rRNA sequence was systematically performed. When a negative result occurred, additional PCR was performed targeting the 18S and 28S rRNA internal transcribed spacer to search for fungal infections. All positive PCR products were sequenced. The sequences were compared to those available in GenBank. Positive PCR results were considered as certain, when congruence existed between the results obtained with PCR and those obtained with other analyses. With a positive result interpreted as a possible case, we performed additional PCR, targeting a second gene with genus-specific primers (Table 1). When the PCR was positive and the sequence gave the same result, the case was reclassified as certain. When the second PCR was negative, we performed a PCR targeting a third gene. When both PCRs targeting the second and the third gene were negative, the result was classified as negative.
Histologic and Immunohistologic Analysis
Thirty-seven valve samples underwent fixation by formalin and were paraffin-embedded. Valve specimens were cut to 3-[micro]m thickness serial sections. Hematoxylin-eosinsaffron, periodic acid-Schiff, Giemsa, Brown-Hopps/Brown-Brenn Gram, Grocott-Gomori methenamine silver, and Warthin-Starry stains were used (16). On the basis of the histologic findings, valve specimens were divided into 3 groups: A, B, and C. Group A samples showed histologic features of infective endocarditis consisting of vegetations or polymorphonuclear leukocyte rich valvular inflammation. Group B specimens showed valvular inflammation composed of mainly inflammatory mononuclear cells, macrophages, and lymphocytes without vegetations and microorganisms. Group C samples were devoid of inflammation, vegetations, or microorganisms. When Bartonella endocarditis was suspected, immunohistochemical analysis was performed on valve sections with an anti-Bartonella rabbit polyclonal antibody as previously described (17).
Serum Sample Analysis
Serologic Testing
Brucella serologic analysis was performed by Rose-Bengale agglutination (Bio-Rad, Marnes-La-Coquette, France) for 61 serum samples from 61 patients in Algiers, and the samples were stored at -20[degrees]C for further study. The confirmation was observed by Wright Serology (Bio-Rad). In the case of endocarditis, specific antibody titers exceeded 1:800. Bartonella and C. burnetii serologic testing was performed in France on all 61 samples. For Bartonella serologic testing, B. quintana and B. henselae were used as antigens in a microimmunofluorescence (MIF) assay performed as previously described (18). A patient was considered to have Bartonella endocarditis when IgG titers [greater than or equal to] 1:800 were observed (18). The species identification was performed with Western blot performed before and after serum cross-adsorption as previously described (19). For C. burnetii serologic testing, immunoglobulin (Ig) G, IgM, and IgA antibody titers were estimated by using an MIF test as previously described (20). A diagnosis of chronic endocarditis was made when a patient had an IgG phase I titer [greater than or equal to] 1:800 (20). A Light Cycler nested PCR was performed on positive serum samples for Bartonella and C. burnetii as previously described (21,22).
Results
Patient Characteristics
Our prospective study led to identification of 110 cases from 108 patients. The 110 episodes were classified as 77 (70%) definite infective endocarditis and 33 (30%) possible infective endocarditis (12). A second episode of infective endocarditis developed in 2 patients during our survey. The patients included 64 men and 40 women with a mean age of 35.3 years (range 17-72 years). The series included 4 children, 2 boys (6 and 8 years of age) and 2 girls (9 and 14 years of age). Among the patients, 34 came from rural areas, 61 lived in urban areas, 1 was in prison, and no information could be obtained for 12. Among 96 patients whose living conditions were known, 59 (61.5%) lived in poor and crowded families of at least 10 persons. Among the 110 cases, 87 (79%) episodes were diagnosed on native valve and 23 (21%) on prosthetic valve. The mitral valve was affected in 31 (28.2%) cases, the aortic in 29 (26.3%), and both in 41 (37.2%). The tricuspid valve was affected in 3 (2.7%) patients, and 4 (3.6%) had aortic, mitral, and tricuspid involvement. We reported 1 case with mitral and pulmonary valves affected, with the persistence of an arterial canal, and 1 patient on a pacemaker.
Blood Cultures
Blood cultures identified 48 microorganisms (Table 2). Of the 22 Streptococcus spp. cultures, 5 Streptococcus mitis, 6 Streptococcus sp., 3 S. agalactiae, 3 Granulicatella adiacens, 2 [alpha]-Streptococcus, 1 S. oralis, 1 S. intermedius, and 1 Gemella morbillorum were identified. Seven Staphylococcus aureus and 5 coagulase-negative Staphylococcus were observed. One Haemophilus influenzae, 1 H. aphrophilus, 1 Haemophilus sp., 1 Kingella kingae, and 1 Actinobacillus actinomycetemcomitans were identified among the HACEK group (Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, Kingella.). One Brucella melitensis, a zoonotic agent, was isolated.
Serum Analysis
Using serologic testing, infective endocarditis could be diagnosed in 11 (18%) of 61 serum samples. A positive Brucella serologic result with titers of 1:3,200 was observed for 2 patients (1 sample was also culture positive). Two other patients had a typical profile of Q fever endocarditis (Phase I: IgG 1:3,200; IgM 1:25; IgA 1:1,600/Phase II: IgG 1:6,400; IgM 1:25; IgA 1:1,600 for 1 patient and Phase I: IgG 1:6,400; IgM 1:800; IgA 1:50/Phase II: IgG 1:12,800; IgM 1:800; IgA 1:100 for the other patient). Among these 2 patients, C. burnetii Light Cycler nested-PCR performed on serum samples was positive for the sample from 1 patient. A positive Bartonella serologic result, with IgG [greater than or equal to] 1:800, was observed for 7 patients (Table 3). The Western-blot analysis of the 7 serum samples allowed the specific diagnosis of B. quintana (Figure 2). Of these 7 patients, B. quintana Light Cycler nested-PCR performed on serum samples was positive for 5 patients (Table 3).
[FIGURE 2 OMITTED]
Cardiac Valve Analysis
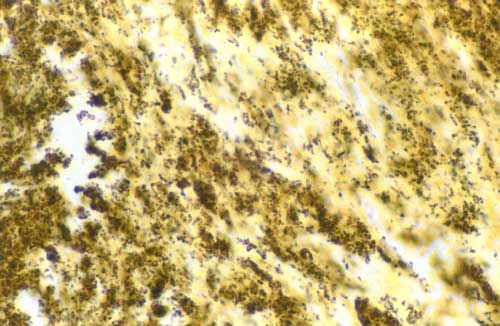
Axenic culture of cardiac valves was positive for 9 samples. The growth of 2 coagulase-negative Staphylococcus, 2 Streptococcus sp., 1 Staphylococcus aureus, 1 Streptococcus mitis, 1 S. intermedius, I Corynebacterium sp., and 1 Candida kruzei was observed. Another sample was polymicrobial. Cell culture allowed the growth of B. quintana, an arthropodborne disease agent, from 3 valve samples (Tables 2 and 3). The numbers of valve specimens classified into groups A, B, and C were 21, 5, and 11, respectively. With the exception of Bartonella endocarditis, the samples with histologic features of infective endocarditis had vegetations in most cases, moderate fibrosis, calcifications in some cases, and numerous inflammatory infiltrates composed predominantly of polymorphonuclear leukocytes and abundant neovascularization. By using special stains, microorganisms were visualized in 16 samples from group A, gram-positive cocci and gram-negative bacilli in 8 cases each. In samples from group B, the inflammatory infiltrates were rare and focal and consisted mainly of macrophages and lymphocytes with discrete neovascularization. The specimens from group C showed noninflammatory degenerative damage with extensive fibrosis and often calcifications. The histologic features of Bartonella endocarditis were different from the other infective endocarditis. Samples from 7 cases with Bartonella endocarditis were examined. The valve tissues showed degenerative damage with extensive fibrosis. The valve tissues were poorly inflamed with rare mononuclear inflammatory cell infiltrates composed of lymphocytes and macrophages and discrete neovascularization. Vegetations, present in all samples, were small in size. In all cases, the Warthin-Starry stain detected Bartonella, mainly in vegetations as small bacillary organisms (Figure 3).
[FIGURE 3 OMITTED]
The 16S rRNA PCR was positive for 29 cardiac valves (Tables 2 and 4). B. quintana was detected on 10 specimens (Table 3). Among the Streptococcus spp. and related genera, 3 Streptococcus sp., 1 S. mitis, 1 S. mutans, 1 S. gordonii, 1 S. pneumoniae, and 1 Granulicatella adiacens were detected. Two Staphylococcus aureus and 1 coagulase-negative Staphylococcus were identified. Among the 2 bacteria from the HACEK group, 1 H. paraphrophilus and 1 Cardiobacterium hominis were identified. PCR performed with a second gene confirmed the previous PCR results with 1 exception. One Streptococcus sp. was not retrieved by PCR targeting a second or third gene and was considered as contamination. The PCR targeting the 18S-28S rRNA ITS allowed the detection of 1 Candida parapsilosis. Finally, Bartonella spp. were also specifically visualized in vegetations by immunohistochemistry in all the cases of B. quintana endocarditis (Figure 3).
Causative Microorganisms and Discordant Results
The overall distribution of causative microorganisms and their identification, depending on the diagnostic tools used, are displayed in Table 2. An etiologic agent could not be determined for 10 (13%) of definite cases and 28 (76%) of possible cases. For the 2 patients with recurring infective endocarditis, the cause for the first episode was different than that of the second episode. One patient had endocarditis caused by Streptococcus oralis, and 1 year later, endocarditis caused by K. kingae developed. For the other patient, no etiologic diagnosis was established for the first episode, during which a valve removal was necessary. Four months after cardiac surgery, the patient had endocarditis caused by Staphylococcus epidermidis. Nine discrepant results were also observed and are summarized in Table 4.
Discussion
Endocarditis cases with fastidious agents escape microbiologic diagnosis classically applied in Algerian laboratories. For the first time, we established a profile of the microbiologic etiology of infective endocarditis in Algeria. Our conclusions concerning PCR results were submitted to a rigorous strategy of validation. All of the controls must be correct for validating each assay. The result was considered true if confirmation was obtained by successfully amplifying bacterial DNA when targeting another gene, the PCR result was congruent with the results of other diagnostic tools, or both.
Of the 77 cases of definite infective endocarditis, the cause was found for 67 (87%) cases. The diagnosis was performed on the basis of positive blood cultures for 44 cases. For 20 (26%) cases, no etiologic diagnosis was obtained in Algeria but was performed in France on the basis of cardiac valve PCR, and Bartonella and Coxiella burnetii serologic testing. These data show improvement in the etiologic diagnosis of endocarditis when molecular or serologic tools are used. The rate of remaining infective endocarditis without cause is comparable to the prevalence in western countries (16). As in other countries, the etiologic distribution is dominated by the bacteria responsible for infective endocarditis, such as Streptococcus spp. and related genera, Staphylococcus spp., and bacteria from the HACEK group. The difference in comparison to other countries is that blood culture-negative endocarditis is mainly linked to zoonotic and arthropodborne agents.
For the 33 cases of possible infective endocarditis, the number of etiologic diagnoses was fewer than those for definitive infective endocarditis However, in this group, some cases are infective endocarditis and others are not. If we consider a Bartonella serologic result [greater than or equal to] 1:800 as a major criterion (5), the 2 possible cases of B. quintana infective endocarditis will be classified as definite. Therefore, Bartonella serologic results should be taken into account in future revisions of the Duke criteria. Of the 48 case-patients with positive blood cultures, 19 had additional samples tested through a second analysis (serologic or molecular methods). Of the 19, 11 had negative results, 5 were concordant, and 3 were discordant. Of these 48 cultures, 1 corresponds to brucellosis.
Of the 62 blood culture-negative endocarditis cases, samples from all were tested by serologic or molecular methods. Of these, 34 were negative, and 28 had an etiologic agent identified. Seventeen of those were due to zoonoses or arthropodborne bacterial diseases.
Discrepancies were observed between the results obtained by using the various techniques. Some discrepancies resulted from culture contamination with the cutaneous flora. A significant rate of contamination has been already reported, and the low specificity of valve culture that we observed confirms these results (23-25). One discrepancy was caused by identification problems at the species level for Streptococcus. This fact has been previously reported (7). Another discrepancy was linked to a Candida species misidentification by phenotypic analysis, which was corrected by using molecular tools. The last discordant case corresponded to a patient for whom blood cultures were positive for H. influenzae. When serum samples were analyzed, a diagnosis of B. quintana endocarditis has been established in the presence of positive Bartonella MIF, Western blot, and PCR. We do not know if B. quintana was misidentified as H. influenzae, which is possible as both are slow-growing, hemin-dependent, small, gram-negative bacteria (26). We believe that as fastidious, small, gram-negative bacteria growing in blood agar, the 2 organisms may be confused.
In Algeria, cases of infective endocarditis caused by zoonotic and arthropodborne disease agents, such as Coxiella burnetii, Brucella melitensis, and Bartonella quintana are frequently observed and correspond to one quarter of the performed diagnoses. B. quintana would be one of the most common agents of infective endocarditis in our Algiers series (15.6% of definite infective endocarditis). The prevalence of endocarditis caused by Bartonella varies depends on the country. In Canada, Bartonella causes 3% of endocarditis cases (27). In Sweden, no Bartonella endocarditis was identified in an analysis of 334 infective endocarditis cases (28). In the United Kingdom, Bartonella endocarditis accounts for 1.1% of infective endocarditis cases (29). In Germany and in France, Bartonella endocarditis accounts for 3% of all infective endocarditis (A. Sander et al. unpub. data) (27). The frequency of Bartonella endocarditis is <1% for Sweden and higher in France, Germany, the United Kingdom (3%), and North Africa (15%). Such differences may be linked to differences in living conditions.
Homeless people are at risk for B. quintana endocarditis (30,31). Indeed, B. quintana, like Rickettsia prowazekii, the agent of epidemic typhus, is transmitted by body lice. Those who live in extreme poverty are often the persons who are infested. The recent description of typhus in Algeria confirms that poor socioeconomic conditions still exist in this country (32-34). In our studies, B. quintana endocarditis cases occurred in patients living in poor conditions. Although the only known reservoir for B. quintana is humans, the bacterium has recently been associated with fleas (35). Moreover, some cases of B. quintana infections have been linked to contact with cats and cat fleas in patients who were not homeless and did not have body lice (36).
Brucella melitensis, well known in northern Africa, where bucellosis is endemic in certain areas, accounts for 2.6% of all infective endocarditis cases for which an etiologic diagnosis has been performed (37). Be cause C. burnetii detection requires specialized tests not normally found in most laboratories, it is not often diagnosed in Algeria (38). Two cases were retrospectively detected.
Importance of infective endocarditis caused by zoonotic and arthropodborne agents in Algeria leads to 2 considerations. First, specific serologic tests need to be used for diagnosis. Indeed, 25% of our etiologic diagnoses correspond to microorganisms for which the diagnosis is usually based on serologic testing. Secondly, the therapeutic impact of Brucella and Coxiella diagnosis is important because the antimicrobial treatment of endocarditis caused by these agents must include doxycycline. The 2 patients with Q fever endocarditis died during their hospitalization because of inadequate antimicrobial therapy. Finally, the high rate of blood culture-negative endocarditis was not linked to prior antimicrobial therapy but rather to fastidious microorganisms for which serologic testing (as for zoonotic and arthropodborne disease agents) or molecular analysis (as for Mycoplasma hominis [39] and Corynebacterium spp.) are diagnostic tools.
Our study underlines the need to perform serologic analysis to determine for the etiology of infective endocarditis. Bartonella serologic testing is an important tool for diagnosis of blood culture negative endocarditis and should be taken into account in future revisions of the Duke criteria. This study made it possible to show that zoonotic and arthropodborne disease agents cause one quarter of infective endocarditis in Algeria; B. quintana caused 13% of our cases.
Acknowledgments
We thank Kelly Johnston for reviewing the manuscript.
Dr. Benslimani is a physician working at the E.H.S. Dr Maouche, Algiers, Algeria. Her research interests include the clinical features and diagnosis of endocarditis.
References
(1.) Moreillon P, Que YA. Infective endocarditis. Lancet. 2004;363:139-49.
(2.) Kezzal K. Importance of blood culture in septicemia, particularly bacterial endocarditis. Arch Inst Pasteur Alger. 1986;55:41-60.
(3.) Bentley S, Maiwald M, Murphy L, Pallen M, Yeats C, Dover L, et al. Sequencing and analysis of the genome of the Whipple's disease bacterium Tropheryma whipplei. Lancet. 2003;361:637-44.
(4.) Gauduchon V, Chalabreysse L, Etienne J, Celard M, Benito Y, Lepidi H, et al. Molecular diagnosis of infective endocarditis by PCR amplification and direct sequencing of DNA from valves tissue. J Clin Microbiol. 2003;41:763-6.
(5.) Rolain JM, Lecam C, Raoult D. Simplified serological diagnosis of endocarditis due to Coxiella burnetii and Bartonella. Clin Diagn Lab Immunol. 2003;10:1147-8.
(6.) Watkin RA, Lang S, Lambert PA, Littler WA, Elliott TSJ. The microbial diagnosis of infective endocarditis. J Infect. 2003;47:1-11.
(7.) Podglajen I, Bellery F, Poyart C, Coudol P, Buu-Hoi A, Bruneval P, et al. Comparative molecular and microbiologic diagnosis of bacterial endocarditis. Emerg Infect Dis. 2003;9:1543-7.
(8.) Goldenberger D, Kunzli A, Vogt P, Zbinden R, Altwegg M. Molecular diagnosis of bacterial endocarditis by broad-range PCR amplification and direct sequencing. J Clin Microbiol. 1997;35:2733-9.
(9.) Mylonakis E, Calderwood S. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-30.
(10.) Millar BC, Moore JE. Emerging issues in infective endocarditis. Emerg infect Dis. 2004; 10:1110-6.
(11.) Millar BC, Moore JE. Current trends in the molecular diagnosis of infective endocarditis. Eur J Clin Microbiol Infect Dis. 2004;23:353-65.
(12.) Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633-8.
(13.) Raoult D, Vestris G, Enea M. Isolation of 16 strains of Coxiella burnetii from patients by using a sensitive centrifugation cell culture system and establishment of the strains in HEL cells. J Clin Microbiol. 1990;28:2482-4.
(14.) La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol. 1999;37:1899-905.
(15.) La Scola B, Michel G, Raoult D. Use of amplification and sequencing of the 16S rRNA gene to diagnose Mycoplasma pneumoniae osteomyelitis in a patient with hypogammaglobulinemia. Clin Infect Dis. 1997;24:1161-3.
(16.) Lepidi H, Durack DT, Raoult D. Diagnostic methods current best practices and guidelines for histologic evaluation in infective endocarditis. Infect Dis Clin North Am. 2002;16:339-61.
(17.) Lepidi H, Fournier P, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. A case control study. Am J Clin Pathol. 2000;114:880-9.
(18.) Fournier P, Mainardi J, Raoult D. Value of microimmunofluorescence for the diagnosis and follow-up of Bartonella endocarditis. Clin Diag Lab Immunol. 2002;9:795-801.
(19.) Houpikian P, Raoult D. Western Immunoblotting for Bartonella endocarditis. Clin Diag Lab Immunol. 2003;10:95-102.
(20.) Tissot-Dupont H, Thirion X, Raoult D. Q fever serology: cutoff determination for microimmunofluorescence. Clin Diag Lab Immunol. 1994;1:189-96.
(21.) Zeaiter Z, Fournier P, Greub G, Raoult D. Diagnosis of Bartonella endocarditis by a real-time nested-PCR assay using serum. J Clin Microbiol. 2003;41:919-25.
(22.) Fournier P, Raoult D. Comparison of PCR and serology for the diagnosis of acute Q lever. J Clin Microbiol. 2003;41:5094-8.
(23.) Giladi M, Szold O, Elami A, Bruckner D, Johnson BL Jr. Microbiological cultures of heart valves and valve tags are not valuable for patients without infective endocarditis who are undergoing valve replacement. Clin Infect Dis. 1997;24:884-8.
(24.) Chuard C, Antley CM, Reller LB. Clinical utility of cardiac valve Gram stain and culture in patients undergoing native valve replacement. Arch Pathol Lab Med. 1998;122:412-5.
(25.) Campbell WN, Tsai W, Mispireta LA. Evaluation of the practice of routine culturing of native valves during valve replacement surgery. Ann Thorac Surg. 2000;69:548-50.
(26.) Colson P, Lebrun L, Drancourt M, Boue F, Raoult D, Nordmann P. Multiple recurrent bacillary angiomatosis due to Bartonella quintana in an HIV-infected patient. Eur J Clin Microbiol Infect Dis. 1996;15:178-80.
(27.) Raoult D, Foumier P, Drancourt M, Marrie T, Etienne J, Cosserat P, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125:646-53.
(28.) Werner M, Fournier PE, Andersson R, Hogevik H, Raoult D. Bartonella and Coxiella antibodies in 334 prospectively studied episodes of infective endocarditis in Sweden. Scand J Infect Dis. 2003;35:724-7.
(29.) Lamas CC, Eykyn SJ. Blood culture negative endocarditis: analysis of 63 cases presenting over 25 years. Heart. 2003;89:258-62.
(30.) Klein JL, Nair SK, Harrison TG, Hunt I, Fry NK, Friedland JS. Prosthetic valve endocarditis caused by Bartonella quintana. Emerg Infect Dis. 2002;8:202-3.
(31.) Posfay Barbe K, Jaeggi E, Ninet B, Liassine N, Donatiello C, Gervaix A, et al. Bartonella quintana endocarditis in a child. N Engl J Med. 2000;342:1841-2.
(32.) Niang M, Brouqui P, Raoult D. Epidemic typhus imported from Algeria. Emerg Infect Dis. 1999;5:716-8.
(33.) Birg ML, La Scola B, Roux V, Brouqui P, Raoult D. Isolation of Rickettsia prowazekii from blood by shell vial cell culture. J Clin Microbiol. 1999;37:3722-4.
(34.) Mokrani K, Fournier PE, Dalichaouche M, Tebbal S, Aouati A, Raoult D. Epidemic typhus is a reemerging threat in Algeria. J Clin Microbiol. 2004:42:3898-900.
(35.) Rolain JM, Franc M, Davoust B, Raoult D. Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerg Infect Dis. 2003;9:338-42.
(36.) Fournier P, Lelievre H, Eykyn S, Mainardi J. Marrie T, Bruneel F, et al. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine. 2001;80:245-51.
(37.) Memish ZA, Balkhy HH. Brucellosis and international travel. J Travel Med. 2004;11:49-55.
(38.) Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518-53.
(39.) Fenollar F, Gauduchon V, Casalta JP, Lepidi H, Vandenesch F, Raoult D. Mycoplasma endocarditis: two case reports and a review. Clin Infect Dis. 2004;38:e21-4.
Address for correspondence: Didier Raoult, CNRS UMR 6020, Unite des Rickettsies, IFR, 48 Universite de la Mediterranee, Faculte de medecine, 27 Boulevard Jean Moulin, 13385 Marseille cedex 5, France; fax: 33-491-83-03 90; email: Didier.Raoult@medecine.univ-mrs.fr
Akila Benslimani, * (1) Florence Fenollar, ([dagger]) (1) Hubert Lepidi, ([dagger]) Didier Raoult ([dagger])
* Service de Biologie Clinique, Alger, Algerie; and ([dagger]) Universite de la Mediterranee, Marseille, France
(1) These 2 authors have contributed equally to the manuscript.
COPYRIGHT 2005 U.S. National Center for Infectious Diseases
COPYRIGHT 2005 Gale Group