Inmates with a history of opiate dependence represent a substantial proportion of the correctional population in the United States. Opiate use has negative consequences for both the inmate and society, including increased recidivism rates, increased infectious disease prevalence, avoidable emergency room use, decreased access to primary care services, and overdose. While there have been great successes in community-based treatment of opiate dependence, these successes have not yet been achieved in correctional settings. This paper reviews the pharmacological treatment options for opiate-dependent inmates, along with potential application for community-to-correctional approaches. The recent approval by the Food and Drug Administration (FDA) of physician-prescribed buprenorphine and the new opportunities it presents to corrections-based treatment are also explored in depth. Successful implementation of such strategies is likely to result in desirable health and social outcomes for both the inmate and the community at large.
OPIATE ABUSE, INCARCERATION, ANDADVERSE HEALTH OUTCOMES IN THE UNITED STATES
Opiate dependence is a severe and growing public health problem in the U.S. It is estimated that almost 900,000 Americans are currently opiate dependent, with heroin dependence being the most common (Kreek & Vocci, 2002). Over 146,000 new individuals began using heroin in 2000, a number that continues to increase (Substance Abuse and Mental Health Services Administration [SAMSHA], 2001). The individual and societal costs of untreated substance abuse are enormous. These costs include overuse of hospital emergency departments, death due to overdose, high unemployment, illegal activity, and incarceration (Mark, Woody, Juday, & Kleber, 2001; Wall et al, 2000). Opiate users, especially injection drug users, tend to be among society's most disease-burdened individuals, with a high prevalence of infectious diseases - most importantly, HIV, hepatitis B and C, and tuberculosis (Edlin, 2002; Garfein, Vlahov, Galai, Doherty, & Nelson, 1996; Hagan et al., 2002; Martin, Cayla, Bolea, & Castilla, 2000; Spaulding, Greene, Davidson, Schneidermann, & Rich, 1999) - and comorbid psychiatric conditions (Milby et al., 1996). Mortality rates among heroin injectors are between six to 20 times higher than their drug-free peers (Sporer, 2003). Furthermore, these medical consequences of opiate use - infectious diseases, mortality, and emergency department use - have increased in recent years (National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction, 1998). The overall economic costs of opiate addiction, especially to poor urban communities, are tremendous. Annual losses due to medical care, lost productivity, crime, and social welfare of heroin abuse alone cost the U.S. $21.9 billion (Mark et al, 2001).
Throughout the 1980s, in an effort to combat the increasing prevalence of substance abuse, many state and federal governments enacted stringent anti-idrug laws. Partially as a result of these measures, incarceration rates in the U.S. have dramatically increased (Beck, Karberg, & Harrison, 2002) and have imposed pressures on a system ill-prepared to address the medical and social consequences of substance abuse (Pollack, Khoshnood, & Altice, 1999). According to the Bureau of justice Statistics, 82% of jail inmates and 83% of state prisoners have a history of substance abuse; 64% and 70%, respectively, use drugs "regularly" (at least once a week for at least a month) in the period immediately preceding incarceration (Hammett, Harmon, & Rhodes, 2002; Harlow, 1998). In regard to specific opiate use, 9% of all state prisoners and jail inmates were using in the month prior to incarceration, and 12% of jail inmates and 15% of state prisoners have used opiates regularly at some point (Chaiken, 2000; Harlow). While the high rates of drug-related arrests, recidivism, and the large numbers of substance abusers within the correctional system are alarming, they also represent an important public health opportunity (Glaser & Greifinger, 1993). Due to the high number of opiate-dependent patients that enter and reenter the nation's prisons and jails, the correctional system is one setting where access to necessary opiate treatment can be greatly expanded. Additionally, the structured environment of the correctional setting can be an ideal place to initiate drug treatment.
Such interventions have thus far not been implemented nor evaluated sufficiently. Currently only 32% of state prisoners and 36% of federal prisoners with substance abuse problems receive any form of treatment while in prison (Mumola, 1999). Interview-based data suggest that a large population of inmates desire treatment in prison but are unable to access it (Brooke, Taylor, Gunn, & Maden, 1998). On the outside, only 15% of opiate-dependent patients presently receive medically-indicated pharmacological treatment (Fiellin & O'Connor, 2002; Kreek & Vocci, 2002; Sporer, 2003). This is counterproductive for the criminal justice system; in the absence of prison-based treatment and linkage to community care following release, drug abuse and recidivism rates will remain high (Langan & Levin, 2002). Interventions initiated in corrections, and continued into the community, could reduce recidivism and address the many psychosocial and medical problems that both inmates and society face as a result of opiate use and dependence.
The recent approval of buprenorphine (BUP) in October 2002 for opiate maintenance therapy provides a new medical option to counter this lack of access. Because of its pharmacological properties and the legal framework under which it is regulated, BUP will provide a unique opportunity to work within the correctional system to address opiate dependence. In order to incorporate BUP into the existing opiate treatment pantheon, it will be useful to review past experiences with the other pharmacological options for opiate dependence. As such, this paper will analyze previous research on the medical treatment of opiate dependence, with the hope of informing future policy and clinical research questions on this new drug. Emphasis will be placed on research that reports on the effectiveness of pharmacological treatments in the community and in correctional settings. Corrections-to-community programs, the required psychosocial services for these therapies, and the strengths and weaknesses of each treatment for different types of inmates will also be explored.
CURRENT TREATMENT OPTIONS FOR OPIATE DEPENDENCE
Multiple therapeutic modalities have demonstrated effectiveness for the treatment of opiate dependence. These modalities can be classified into two broad classes: drug-free therapeutic communities (TC) and pharmacological interventional therapies (methadone, LAAM, naltrexone, and buprenorphine). It is beyond the scope of this review to discuss in-depth the TC approach in the prison setting, which has been subject to extensive study (Butzin, Martin, & Inciardi, 2002; Killer, Knight, & Simpson, 1999; Knight, Simpson, & Killer, 1999; Nielsen, Scarpitti, & Inciardi, 1996; Wexler, Melnick, Lowe, & Peters, 1999). Briefly, traditional therapeutic communities (TC) are residential, long-term (six to 12 months and longer) programs that provide the behavioral and psychosocial skills necessary to remain abstinent from drugs. Central to this philosophy, pharmacological treatments are discouraged and often viewed as enabling. TCs primarily focus on the teaching of "living right" and emphasize responsibility for self and others, (De Leon, 1996) in an attempt to achieve "lasting lifestyle changes" (Nielsen et al., 1996). Perhaps the single greatest value of TCs is that they work towards getting the client off all drugs and for this reason can treat comorbid cocaine and alcohol addiction and can avoid medication dependence that can develop in pharmacological programs.
A few generalizations can be made regarding situations that might be beneficial for prison-based TCs: (1) inmates with prolonged sentences who therefore have time to spend in an intensive program; (2) correctional systems with resources to fund comprehensive TC-based services linked to adequate aftercare programs; (3) individuals who are highly motivated (De Leon, Melnick, Kressel, & Jainchill, 1994; Wexler et al., 1999); (4) individuals with a history of severe drug abuse (Miller et al., 1999; Knight et al., 1999; Nielsen et al., 1996); and (5) those without co-occurring mental illness or those whose mental illness is adequately treated (Katz, 1999; Milby et al., 1996). Motivation of the staff, cooperation of prison authorities, increasing levels of client responsibility, consensus-based decision making, and provision of aftercare are all also central to success of TC-based programs (Jones, 1980; Rouse, 1991). Additionally, in terms of both recidivism and relapse rates and cost effectiveness, linkage to community programs upon release is central to the success of such prison-based TCs (Chanhatasilpa, MacKenzie, & Hickman, 2000; Griffith, Killer, Knight, & Simpson, 1999; Hiller et al., 1999; Knight et al., 1999; Nielsen et al., 1996). These considerations will also be important in assessing where and how to implement pharmacological interventions in the correctional setting.
Unlike prison-based TCs, there is generally little experience with or utilization of pharmacological treatments for opiate-dependent correctional inmates. This is primarily related to the logistical failures of such programs in the 1970s and the increased societal demands to reduce "coddling" of criminal offenders. Correctional administrators themselves tend to favor drug-free options such as TC-based programs. Evaluations of prison-based interventions that do make it past these logistical hurdles are plagued by difficulties in standardizing treatment protocols, including dosages, counseling techniques, social services provisions, and the environment of treatment delivery. Most research to date in this realm has generally been process- rather than outcome-oriented. Moreover, appropriate control groups are often lacking. For these reasons, sufficient data from well-controlled studies are not yet available for a systematic comparison of the different treatments. The following sections will present some preliminary data on the effectiveness of the various interventions and some of the barriers to their successful implementation in the community and in corrections. Emphasis will be placed on outcome-oriented research of interventions that work on a continuum model of prison-to-community treatment.
NALTREXONE
Naltrexone, a long-acting, pure opiate antagonist that competitively inhibits the euphoric effects of opiates, has been in use for the treatment of opiate addiction for decades (Farren, O'Malley, & Rounsaville, 1997). Atypical naltrexone regimen is 100 mg Monday and Wednesday and 150 mg on Friday, although 50 mg daily and twice weekly 100/150 mg have also been studied (Kirchmayer, Davoli, & Verster, 2002). Treatment initiation generally requires an effective detoxification of at least five to seven days prior to treatment initiation to prevent the precipitation of severe withdrawal. The efficacy and safety of naltrexone for the treatment of opiate dependence has been demonstrated in several randomized, controlled clinical trials (Gonzalez & Brogden, 1988).
The major strength of this method is that there are no opiate-related side effects, no overdose risk, and no possibility for diversion. Additionally, naltrexone has some beneficial effects in the treatment of moderate alcoholism (Chick et al., 2000; Feeney, Young, Connor, Tucker, & McPherson, 2001), a common comorbid condition among opiate users. Naltrexone's effectiveness has been hampered by decreased adherence because, unlike methadone, there is neither positive reinforcement for continued use (e.g., decreased pain perception) nor negative consequences upon cessation (e.g., withdrawal). Some reports have also suggested that naltrexone may cause dysphoria, possibly as a result of the blocking of endogenous opiates (Crowley, Wagner, Zerbe, & Macdonald, 1985). Hence, the effectiveness of naltrexone heavily depends upon the motivation and social support system of the patient (Greenstein, Evans, McLellan, & O'Brien, 1983). Indeed, the drug is most effective among "white collar" patients, such as opiate-dependent health professionals, and has achieved its best results when treatment was contingent upon continued employment (Roth, Hogan, & Farren, 1997; Washton, Gold, & Pottash, 1984). Systematic metaanalysis indicates that naltrexone is no better than a placebo except when used in combination with behavioral therapy, and this effect was explained primarily by subject motivation (Kirchmayer, Davoli, Verster et al., 2002).
Naltrexone was first used in the U.S. among incarcerated populations as part of a work-release program, involving 691 work-release inmates in Nassau County, New York (Brahen, Henderson, Capone, & Kordal, 1984). While the program was not a controlled experiment with no outcomes described for the subjects, the correctional officials, clients, and physicians involved viewed naltrexone favorably. Subsequently, a pilot study was conducted among federal parolees (Cornish et al., 1997). Naltrexone therapy was stipulated as a condition for parole. Parole officers directly observed naltrexone administration, tested the urine weekly for opiates, and coupled treatment with parole in 51 subjects. Using historical controls, both retention in treatment (52% in treatment versus 33% in control) and mean opiate positive urine test (8% versus 30%) were improved. Notwithstanding these preliminary results, randomized controlled trials with appropriate controls and longer follow-up beyond the period of parole are necessary to determine their long-term effectiveness. The effectiveness of such programs depends on prisoners who have probation or parole stipulations, the duration of the stipulation and the degree to which parole or probation officers are co-trained in the area of drug treatment. Still, especially for the highly motivated subject under a structured environment, naltrexone remains a viable option.
Recently, phase II clinical trials have been begun to evaluate a longer-acting naltrexone formulation that confers effects to the subjects for up to one month (Comer et al., 2002; Modesto-Lowe, 2002); this may expand naltrexone's applicability in both community and correctional settings. For example, it might be administered soon after incarceration for unsentenced inmates and immediately prior to release for sentenced prisoners. As such, it may allow for a reprieve from immediate recidivism to opiate use and prevent overdose while social factors are stabilized. However, such applications await successful completion of Phase II and III trials.
METHADONE
Methadone is a full opiate agonist with a long half-life of 12-36 hours that can be administered once daily because of its relatively constant plasma levels over a 24-hour period. Daily dosing regimens are variable and patient-specific, although larger doses, on the order of 80-100 mg, are overall more effective than 40-50 mg in reducing illicit opiate use (Strain, Bigelow, Liebson, & Stitzer, 1999). This may be because lower doses suppress heroin withdrawal symptoms, but higher doses block the opiate receptor, such that heroin provides no effect (Donny, Walsh, Bigelow, Eissenberg, & Stitzer, 2002). Methadone does not provide, when properly dosed in stabilized patients, a euphoric sensation because peak levels are attenuated compared to heroin, with methadone taking two to six hours to obtain peak levels. Dependency develops rapidly, and missed doses result in severe withdrawal symptoms (Liu & Wang, 1984).
Forty years of experience and extensive research in the U.S. have demonstrated the cost-effectiveness of methadone at increasing retention in treatment, decreasing heroin use, and reducing crime and HIV risk behaviors (Marsch, 1998; Mattick, Breen, Kimber, & Davoli, 2002; Yoast, Williams, Deitchman, & Champion, 2001; Zaric, Barnett, & Brandeau, 2000). Nevertheless, in the United States and many countries, community-based methadone treatment clinics are strictly regulated and rarely are able to meet treatment demand (Rettig & Yarmolsky, 1995). Regulation is central in reducing diversion to illicit use, but this practice severely limits access to treatment because of the small number of funded treatment slots. Recently, attempts to expand access have demonstrated considerable success by transitioning stable methadone-maintained patients from methadone clinics to physician-prescribed treatment (Fiellin et al., 2001). It is still unclear where the balance between limiting diversion and increasing access might lie. However, one recent report from the United Kingdom, where expanded access policies in which methadone is made available through prescription by general practitioners, demonstrated that fewer deaths result from methadone than from heroin (Hickman et al., 2003). This suggests that the U.S. system has placed a greater emphasis on preventing methadone-related deaths at the expense of preventing heroin-related deaths.
There have been several experiments worldwide applying methadone maintenance treatment to prison populations. In Canada, early success in the reduction of illicit drug use among methadone maintenance treatment program (MMTP) participants in provincial prison (Rothon, 1997), was followed by a Correctional Service of Canada-sponsored program to provide methadone maintenance for opiate-addicted federal prisoners in 1998 (Sibbald, 2002). Initially, incarcerated individuals who were enrolled in community-based MMTP were allowed to continue their treatment while in prison. The success of the program and the lack of diversion resulted in a significant policy change in May 2002, such that all opiate-addicted prisoners are now provided methadone treatment. In 1987, New South Wales, Australia initiated a prison-based methadone treatment program that has since gained widespread acceptance by correctional officers, medical staff, and inmates (Byrne & Dolan, 1998), in part because it has was shown to reduce injection drug use practices in prison (Dolan, Hall, & Wodak, 1996). Unfortunately, none of these programs have been rigorously evaluated for effectiveness in reducing crime and illicit use.
Despite the overall inexperience with prison-based MMTP in the United States, one model program in New York's Riker's Island, Key Extended Entry Program (KEEP), has been implemented and evaluated since 1987 (Tomasino, Swanson, Nolan, & Shuman, 2001). In order to address the concerns of correctional officials regarding diversion to illicit use, Project KEEP used community-based strategies such as directly observed therapy (DOT) techniques using a public health nurse and correctional officer. This has minimized diversion in this setting (Tomasino et al.). The lack of diversion and reduction of "difficult" behaviors of inmates experiencing opiate withdrawal have resulted in the acceptance of methadone maintenance by correctional officers and administrators.
Project KEEP's success is dependent on the linkage between the prison- and community-based MMTPs that provide a continuum of care between the community and prison. As such, patients entering the jail already on methadone are maintained. The program also offers methadone initiation or opiate detoxification for opiatedependent patients who are not already on pharmacological treatment. Upon release, patients may choose between continued methadone or levo-alpha-acetyl-methadol (LAAM) treatment; 20% chose the latter.
Successful outcomes for this program include linkage to continued MMTP in the community (74-80%) (Tomasino et al., 2001), a higher linkage to community-based drug treatment programs than those who underwent opiate detoxification (85% vs. 37%), and a decrease in injection drug behaviors at six months postrelease (70% vs. 44%) (Magura, Rosenblum, Lewis, & Joseph, 1993). Despite the KEEP Program successfully linking more patients to community drug treatment, the six-month retention was modest; 27% of KEEP vs. 9% of opiate detoxified patients remained in treatment (Magura et al.). Successful linkage to and retention in drug treatment after prison release varied among individuals within KEEP. Participants who were on methadone prior to incarceration and methadone-naive participants who were placed on higher methadone doses (>30 mg/day) fared best (Tomasino et al.). This latter finding is in keeping with the several studies that have demonstrated that patients on higher dose methadone do better than those on a lower dose (Dole, Nyswander, & Kreek, 1966; Donny et al., 2002; Strain et al., 1999). Thus, having adequate resources - facilities, staffing, and especially medications - in the prison setting is vital to linking inmates to community-based treatment.
In sum, methadone maintenance is gradually gaining acceptance in several countries as a viable option for treatment among opiate-dependent inmates. Perhaps the most important rationale for expanded methadone programs in the correctional system is for those inmates already on methadone in the community prior to incarceration. A major risk factor for the use of illicit drugs within prison is related to a failure to continue methadone maintenance treatment that the inmate had been receiving in the community prior to incarceration (Bird, Gore, Cameron, Ross, & Goldberg, 1995; Vormfelde & Poser, 2001). Withdrawal symptoms due to forced abstinence from methadone following incarceration are a major source of negative attitudes towards methadone among injection drug users (Zule & Desmond, 1998). Expanded access to MMTPs for methadone participants in the correctional setting could improve this problem. The political realities that have hindered methadone acceptance in U.S. correctional facilities are important to consider in analyzing the feasibility of novel therapies, such as buprenorphine, for corrections-based substitution treatment.
LAAM
Levo-Alpha-Acetylmethadol (LAAM) is similar to methadone as a full opiate agonist, but its longer half-life allows for thrice weekly administration. In randomized clinical trials, it has been found to be equivalent to methadone in reducing opiate use, decreasing injection and improved social functioning (Clark, Lintzeris, Gijsbers et al., 2002; Kleber, 2003). Current Drug Enforcement Administration (DEA) classification as a Class II controlled substance presents similar regulatory hurdles as does methadone. The drug's onset of action is longer than methadone and therefore less immediately reinforcing to patients; hence, many patients prefer methadone. Its more convenient dosing regimen, on the other hand, makes it attractive. Unlike methadone, LAAM was found in post-marketing treatment to be associated with prolonged QTc interval and torsade de pointes, thus relegating this therapy as second-line opiate replacement therapy (Kinlock, Battjes, & Schwartz, 2002). LAAM is metabolized preferentially, but not solely, by hepatic cytochrome P450 isoform CYP3A4 (Neff & Moody, 2001), which is also responsible for the metabolism of many other pharmaceuticals including protease inhibitors, anti-epileptic drugs and antibiotics (Oda & Kharasch, 2001). Such pharmacological interactions potentially increase the risk of arrhythmia, however uncommon, and further limit its usefulness and acceptance among patients.
LAAM has not been implemented much in the correctional setting; most likely its greatest use will be as an alternative to methadone in MMTPs, as it is in the Project KEEP program. One pilot study among 58 prisoners in Baltimore attempted to initiate LAAM maintenance therapy in prison and continue the treatment upon release (Kinlock et al., 2002). Unlike KEEP, this program focused on a longer-term prison population as opposed to jail inmates who remain incarcerated less than one year and often for only a few days. Due to regulatory and diversion concerns voiced by correctional officials about storing LAAM on-site, the community health clinic providing post-release LAAM delivered and administered the medications. This approach aided continuity of care because the same health providers and clinic treated patients in the correctional and community setting. Among eligible inmates, interest in participation exceeded 90% and, similar to KEEP, continuity after community-release exceeded 80%; six-month retention was 50%. Like methadone and TC, maintaining patients in treatment is a central but often difficult problem.
While this pilot study suggests a role for LAAM, it is unlikely that it will ever gain widespread use due to its undesirable pharmacological properties. The less-than-daily dosing does make it attractive, however, and should be considered as an option in prison- or community-based treatment programs where methadone or other pharmacological treatment is not feasible. It should also be an option, as it is in Project KEEP, among appropriate patients desiring maintenance therapy who choose LAAM over methadone.
BUPRENORPHINE
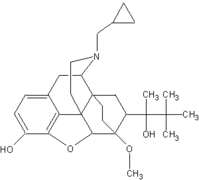
Buprenorphine (BUP), long-used in pain management, has been studied since 1978 as an opiate addiction medication (Jasinski, Pevnick, & Griffith, 1978). Unlike full agonists like methadone and LAAM, buprenorphine is a partial m-receptor agonist. As a partial agonist, there is a plateau of its agonist effects at higher doses that enhances its safety profile compared to full agonists and reduces its likelihood for street diversion (Fiellin & O'Connor, 2002; Ling & Smith, 2002). This "ceiling effect" includes an upper limit on the severity of side effects associated with overdose, such as respiratory depression (Liguori, Morse, & Bergman, 1996; Walsh, Preston, Stitzer, Cone, & Bigelow, 1994). As with methadone, a potential for abuse exists (Tzschentke, 2002). To combat this potential for abuse, buprenorphine is marketed in the U.S. alone (Subutex) and in combination with naloxone (Suboxone). Naloxone, when administered sublingually, has limited bioavailability. However, when crushed and injected, it precipitates acute opiate withdrawal, a potent negative reinforcement for that behavior. Buprenorphine itself, at high doses, can precipitate opiate withdrawal because it binds to the m-receptor with greater affinity than heroin, thus dislodging the heroin (Clark, Lintzeris, & Muhleisen, 2002; Schuh, Walsh, Bigelow, Preston, & Stitzer, 1996). Finally, BUP dissociates slowly from the m-receptor, allowing for alternate day dosing (Fudala, Jaffe, Dax, & Johnson, 1990; Johnson et al., 1995).
Several randomized, controlled trials have demonstrated BUP's efficacy in managing opiate withdrawal (Gowing, Ali, & White, 2002; Mattick, Kimber, Breen, & Davoli, 2002) and opiate dependence (Doran et al., 2003; Johnson et al., 2000). These studies led to U.S. FDA approval of Suboxone and Subutex in October of 2002. Additionally, BUP's pharmacological properties compelled the DEA to classify BUP as a Class III controlled substance, thereby allowing its prescription by properly trained generalist practitioners under the Drug Addiction Treatment Act of 2002. This should greatly expand access to BUP maintenance therapy by avoiding the need for the tightly regulated, specialist clinics that have plagued the expansion of MMTPs. France approved physician-prescription of BUP in 1996, and the Australian government has also begun implementing a national buprenorphine program.
Since a continuity-of-care program following incarceration will require community-based treatment upon release, an examination of studies in community settings is informative for the development of future correctional-to-community interventions. While the drug's efficacy has been proven through numerous clinical trials, it has been subject to fewer tests of effectiveness in real-world settings. France has had the most experience with community-based BUP treatment; within a few years after its approval in 1996, the number of BUP maintenance patients exceeded that of methadone by a factor of 10 (Auriacombe, Franques, & Tignol, 2001). One prospective study of 105 community-based French physicians and 909 opiate-dependent patients showed improvement in housing, employment, and social status and self-reported heroin and other illicit drug intake; low HIV, HBV, and HCV seroconversion was also demonstrated (Fhima, Henrion, Lowenstein, & Charpak, 2001).
There were, however, several problems with the widespread introduction of BUP in France. BUP was used illicitly, primarily by injection, resulting in unanticipated morbidity and mortality (Claudon-Charpentier, Hoibian, Classer, Lalanne, & Pasquali, 2000). Overdoses and death occurred when co-administered with benzodiazepines that are commonly injected in Europe but not the U.S. These anecdotal reports have led the manufacturer to caution the use of BUP in patients taking benzodiazepines (Obadia, Perrin, Feroni, Vlahov, & Moatti, 2001). Still, the problem of overdose seems to be less so than with methadone; in a review of all cases from 1994 to 1998 in France reported to a centralized illicit drug use agency, methadone use had a three times greater mortality than buprenorphine (Auriacombe et al., 2001). Diversion of BUP is further reduced through the Suboxone co-formulation of BUP with naloxone that, when injected simultaneously, results in opiate antagonist effects which precipitate acute withdrawal (Stoller, Bigelow, Walsh, & Strain, 2001).
The U.S. experience has been more limited because of the more recent approval of BUP. The current regulatory context is more stringent in the U.S. than in France; any group practice in the United States, regardless of the number of trained physicians, is limited to a total of thirty patients. Some pilot data has been recently published on community-based buprenorphine. One pilot study of 46 subjects administered thrice-weekly BUP in a primary care clinic was compared to methadone administered at a specialized MMTP. BUP resulted in higher levels of retention and clean urine toxicology than methadone (O'Connor et al., 1998). One speculation from this study is that primary care clinics are less stigmatized than traditional drug treatment settings and provide more comprehensive services that are often required for a population with multiple co-morbid medical and social problems. A recent smaller study involving 14 patients in a 13-week clinical trial demonstrated that, in combination with a brief counseling intervention, buprenorphine was feasible in the ambulatory primary care setting; 11 patients were retained through the maintenance phase (Fiellin et al., 2002). These preliminary studies suggest that buprenorphine may prove effective as it is implemented in the community.
While corrections-based programs have yet to be implemented in the U.S., the French Ministry of Health has provided buprenorphine to incarcerated injection drug users since 1996 (Durand, 2001), and this represents the longest and largest program internationally. A retrospective cohort study of over 3,600 medical files of French prisoners analyzed the comparative effectiveness of methadone, buprenorphine, and abstinence treatment following the legalization of prison-administered buprenorphine. Compared to abstinence-based treatment, both BUP and MMTP within prison resulted in reduced recidivism rates (Levasseur, Marzo, Ross, & Blatier, 2002). The early successes of the French experience with BUP in corrections highlights the need to apply and evaluate BUP within the U.S. correctional system. The following sections discuss some of the policy and medical aspects that should be considered as these programs are implemented.
IMPLEMENTING BUP IN THE CORRECTIONAL SYSTEM
The recent expansion of BUP to the primary care setting provides a potentially exciting opportunity to revisit the use of opiate substitution therapy within correctional and correctional-to-community settings. The relative lack and high cost of methadone maintenance slots in most communities (Fiellin & O'Connor, 2002; Raisch, Fye, Boardman, & Sather, 2002), the unique pharmacological properties of BUP as a partial opiate agonist, and the less stringent regulation of BUP compared to methadone are compelling reasons to consider BUP substitution therapy for the correctional and transitional settings. Notwithstanding these exciting possibilities, there are several obstacles that will hinder the implementation of BUP in both corrections and the community. These will be discussed, with some analysis of previous practical attempts at surmounting these obstacles.
STRUCTURAL OBSTACLES
Perhaps the most important obstacles, certainly those that have paralyzed expansion of MMTPs, are structural ones relating to acceptability, availability, and access of services (Blankenship, Bray, & Merson, 2000). First, physicians may not fully embrace and gain the necessary skills to prescribe BUP. This may be particularly true for correctional physicians who often lag behind the community with regard to "best practices" (Skolnick, 1998a, 1998b). Second, correctional settings are often ill-equipped to provide psychosocial and medical services necessary for an effective addiction program (see below). Third, the lack of access to and desirability of primary care clinicians among drug-addicted patients released from correctional institutions will hinder linkage-to-care programs. Finally, current federal law limits access on the outside by stipulating that those primary care clinicians willing to see patients released from correctional institutions will be limited to only 30 BUP patients for the entire practice.
Important in gaining political acceptance among the public and correctional officers - and therefore gain the support necessary to fight these structural obstacles - is reducing illicit use of prescribed buprenorphine. This is a real, if sometimes exaggerated, concern. For example, the decline in heroin availability and the clinical practice of prescribing injectable (intramuscular) buprenorphine to heroin addicts led to widespread illicit buprenorphine use in parts of India (Ball, Rana, & Dehne, 1998). In France, buprenorphine-associated overdose has been documented following its approval for general practice prescription there (Claudon-Charpentier et al, 2000). The Suboxone formulation, not available yet in France, will further reduce diversion, by reducing the possibility for illicit injection use (Stoller et al., 2001). A New Zealand study presented evidence of diminished, but not zero, abuse of a buprenorphine-naloxone combination following its introduction in 1991 (Robinson, Dukes, Robinson, Cooke, & Mahoney, 1993).
THE NEED FOR PSYCHOSOCIAL AND HEALTHCARE SERVICES
Opiate-dependent patients have multiple unmet psychosocial needs that place them at high risk for recidivism, relapse, and overdose following release from incarceration (Nurco, Hanlon, & Kinlock, 1991). These same unmet needs increase risk for transmission of HIV, viral hepatitis, and sexually transmitted diseases (Sheu et al., 2002; Thompson et al., 1998). Additionally, pharmacological treatment addressed solely as opiate dependence may fall short due to use of other illicit drugs. For example, in the KEEP study, 25% of the participants were also cocaine users (Magura et al., 1993; Tomasino et al., 2001); nationally, 40% of heroin users may also use cocaine (Hser, Anglin, & Fletcher, 1998). Opiate substitution therapy will substantially reduce opiate-associated behavior that puts the addict at risk for infectious disease, but opiate substitution alone will not ensure safe sex, abstinence from other drugs, involvement in primary care, and improved social habits - activities that would improve infectious- and noninfectious-associated morbidity and mortality. Pharmacological maintenance programs should thus be viewed as a part of the larger promotion for public health and the improved health and psychosocial status of opiate-dependent inmates.
The extent and nature of ancillary psychosocial services are thus essential for successful outcomes, regardless of which treatment is selected for the patient. For example, provision of transportation (Friedmann, Lemon, & Stein, 2001) and contact with state social services (Desland & Batey, 1991) have been shown to play a role in retention in community-based clinics. Across a wide range of treatment modalities, psychiatric care, involvement by family members, employment, and medical services are predictive of positive outcomes (McLellan et al., 1994). Given the high rates of incarceration of people of color in the U.S. correctional system (Beck, Karberg, & Harrison, 2002), cultural considerations (i.e., bilingual and bicultural services) are important (Osemene, Essien, & Egbunike, 2001). Case management services both within and outside correctional facilities are a central component to the treatment of drug abuse, but oftentimes they are hindered by the clients' ability to meet basic needs like shelter and food (Hasson, Grella, Rawson, & Anglin, 1994). These linkage services have shown in retrospective cohort studies to be effective and low-cost interventions that promote short-term retention in treatment and prevent relapse in patients discharged from various treatment programs (Shwartz, Baker, Mulvey, & Plough, 1997).
Healthcare services are also a key in a population with serious medical needs, and access to health care is central to any effective transitional drug treatment intervention (Osemene et al., 2001). Traditional methods to improve access to primary care services among addicted patients, such as community health centers and mobile health care units (Altice, Springer, Buitrago, Hunt, & Friedland, 2003; Kuo et al., 2003; Liebman, Lamberti, & Altice, 2002), will need to be explored as options in maintaining the prison-to-community care continuum for BUP. Once patients are within the healthcare system, it is critical that community clinicians are prepared to decide which patients would benefit from outpatient BUP in the primary care setting and for whom a specialized drug treatment clinic is necessary. A staging system using admission questions, similar to those used in cancer prognosis, has been developed to achieve this objective (Favrat, Rao, O'Connor, & Schottenfeld, 2002).
Despite the clear need for access to ancillary social services among opiateaddicted inmates, such services are often not available or not effective, leading to gross underutilization of these necessary components to treatment (Widman, Platt, Lidz, Mathis, & Metzger, 1997). There is vast variability in the delivery of these ancillary interventions, depending upon site, staff, and patient characteristics (Widman et al., 1997), complicating policy and health care decision-making. This is partially because of the paucity of randomized controlled trials looking at how provision of these services impacts methadone treatment; the findings cited above are primarily the result of questionnaires and cohort studies.
The need for such trials is demonstrated by the few good controlled trials that have been done. One such trial involving MMTP alone, MMTP plus counseling, and MMTP plus medical services, employment, and family counseling, showed a clear gradation of effectiveness depending upon the level of social services provided (McLellan, Arndt, Metzger, Woody, & O'Brien, 1993). However, a cost-effectiveness analysis suggested that money could be better spent by expanding access to traditional MMTPs as opposed to enhancing existing ones through added social services (Kraft, Rothbard, Hadley, McLellan, & Asch, 1997). Another clinical trial of 86 patients receiving group therapy, on-site medication services, weekly counseling, plus methadone versus buprenorphine maintenance showed that buprenorphine had better long-term outcomes (Strain, Stitzer, Liebson, & Bigelow, 1996). This is in comparison to other studies in which methadone clearly had the better long-term effects (Farre, Mas, Torrens, Moreno, & Cami, 2002), although the variance tends to be large (Barnett, Rodgers, & Bloch, 2001 ). These nonintuitive results highlight the need for more rigorous studies of the psychosocial services provided in the context of pharmacological maintenance therapy.
COMMUNICATION AMONG PROVIDERS
Because of the complex needs of opiate-dependent patients, especially those within the correctional system, communication among the different providers is central. In addressing this problem, the Glasgow "shared care" methadone system is instructive. This program consisted of general practitioners who agreed to maintain common treatment standards and attend monthly educational sessions and who were compensated for their extra efforts. Drug counselors and pharmacists were involved to reduce clinician responsibility and to provide the social and psychological support necessary to maintain proper adherence to treatment. Finally, as mentioned above, pharmacy-based supervised self-administration of the methadone helped reduce diversion. This program showed excellent participation and retention of physicians and pharmacists (Gruer et al., 1997). The program resulted in reduced injection practices, opiate use and overdose, crime, and money spent on drugs. This was especially true for individuals that remained in the program for at least 12 months (Hutchinson et al., 2000).
In addition to integrating services among community-based pharmacists, counselors, and physicians, a model of communication between the correctional and community physicians is essential for a program's success. This will require the establishment of a network of community physicians who can accept referrals for treatment with little or no notice as jail detainees and prisoners are released from the correctional setting secondary to commuted sentences, payment of bond, or unanticipated release from court.
WHO STANDS TO BENEFIT?
A "one treatment fits all" approach in correctional settings is not compatible with community standards and will significantly diminish the benefit to the public's health. Therefore, careful attention to the specific correctional environment and assessment of the individual is required to prescribe treatment for correctional inmates within jails and prisons. Appropriate intake assessment of prisoners is essential in designing an appropriate and effective treatment course. The following are central to an initial evaluation of the client: likelihood of release for jail detainees; duration of sentence for prisoners; severity and duration of heroin use; dependence on other drugs; comorbid medical conditions such as mental illness, HIV, or viral hepatitis; levels of social support; and living circumstances after release to the community.
Depending on the detail of the intake information gathered, decisions can be made regarding the optimal course of action for the patient (see appendix for overview). Buprenorphine may well find its niche where prison- or community-based methadone specialty clinics are not feasible and where methadone is not suitable (e.g., short-term opiate dependent patients and adolescents). Alternatively, it may be used when correctional systems are unwilling or unable to comply with the strict regulation required for methadone maintenance. BUP should also be considered as part of an alternative that seeks to reform young and first-time offenders by providing drug treatment and community service in lieu of incarceration.
In order to address drug treatment needs throughout the correctional system, it will be essential to examine the conditions and infrastructure of both jails and prisons. The approaches are likely to be different given the differing populations and time constraints. Jails house pretrial detainees and sometimes prisoners sentenced to less than two years. Thus, the majority of unsentenced detainees will be released from jail within days to weeks, while those who are sentenced serve a median time of nine months (Harlow, 1998). Policy changes for jails are likely to be more erratic because they are usually under the jurisdiction of local communities. In a jail setting, brief structural interventions such as methadone maintenance or buprenorphine therapy might be initiated with the plan to transition to a community-based program or practitioner upon release. Jail inmates are also likely to be younger and to have used psychoactive substances just prior to incarceration than those who reside within the prison system. For these individuals, there is a critical moment where drug treatment opportunities exist before release to the community. This is especially true in the case of new opiate users entering the jail system briefly who have yet to make the transition to injecting heroin use. The possibility for preventing hepatitis C and HIV rests upon the ability to reach opiate abusers early, before they have made the transition to injection drug use and seroconverted (Altice et al., 1998). Buprenorphine may be especially suited, given its pharmacological profile, for this type of intervention among younger users.
The landscape is different for prison inmates. The longer sentences imposed upon prisoners provide an optimal time to address multiple social, psychological, and criminal problems that would otherwise confound drug treatment. Interventions among longer-sentenced prisoners can be less immediate, but they need to be longer-term to be effective. Prison-based TCs may be highly effective with some of these prisoners, but such an approach requires high investment in infrastructure to promote the drug-free environment. This infrastructure must incorporate this goal within the prison throughout the transitional period and plan for aftercare long after release to the community in order to be effective. In the absence of this commitment, pharmacological therapy initiated in prison may serve as an effective conduit to treatment after release for individuals with a high likelihood of relapse.
CONCLUSION: NEW POSSIBILITIES AND OLD BARRIERS TO EFFECTIVE TREATMENT
It is time to examine further the realm of possibilities of drug treatment within the correctional system. The correctional system is in a unique position to efficiently identify and initiate effective treatment. Both the World Health Organization (WHO) and the Institute of Medicine have written that pharmacological maintenance programs should be developed where practical in prisons as a means to reduce opiate use and its severe consequences and to control the spread of AIDS among intravenous drug users (Rettig & Yarmolinsky, 1995; WHO, 1993). Effective drug treatment interventions, particularly pharmacological ones within correctional settings, are an important investment in the public's health. Despite the pressing needs for substance abuse treatment in this country and internationally, treatment expansion has been slow. While therapeutic communities have made great strides in corrections, MMTP implementation in prisons remains noticeably absent, due to lack of acceptance by politicians, the public, and correctional officers, and the logistical difficulties of developing effective and safe programs in correctional facilities.
Recently approved drugs containing buprenorphine open a new avenue for the treatment of opiate dependence in a correctional-to-community program. By reducing diversion to illicit use, increasing availability and acceptance, and reducing stigma, BUP may prove a highly effective tool in reducing crime, infectious disease, and recidivism rates among opiate-dependent inmates. Yet many of the barriers and potential problems that have hindered other treatments are likely to plague buprenorphine. Rigorously controlled clinical effectiveness trials should be pursued to determine the contextual factors that affect buprenorphine treatment programs in corrections and in the community, as well as interventions that impact continuity of care from prison to community. Also needed is research into ways to expand access to BUP through nontraditional avenues to care among drug users. Medical, psychological, drug treatment, and social service professionals will have to lead this dialogue, together with community groups, to educate the public, and to reduce barriers to effective implementation. The hope is that newer and effective treatment modalities will gain acceptance as important public health interventions in both correctional and community settings.
ACKNOWLEDGMENTS
Funding for this research was provided by The National Institute on Drug Abuse (K24-DA17072).
REFERENCES
Altice, F. L., Mostashari, F., Selwyn, P. A., Checko, P. J., Singh, R., Tanguay, S., & Blanchette, E. A.
1998 Predictors of HlV infection among newly sentenced male prisoners. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 18(5), 444-453.
Altice, F. L., Springer, S., Buitrago, M., Hunt, D. P., & Friedland, G. H.
2003 Pilot study to enhance HIV care using needle exchange-based health services for out-of-treatment injecting drug users. Journal of Urban Health, 80(3), 416-427.
Auriacombe, M., Franques, P., & Tignol, J.
2001 Deaths attributable to methadone vs buprenorphine in France. Journal of the American Medical Association, 285(1), 45.
Ball, A. L., Rana, S., & Dehne, K. L.
1998 HIV prevention among injecting drug users: Responses in developing and transitional countries. Public Health Report, 113 Suppl 1, 170-181.
Barnett, P. G, Rodgers, J. H., & Bloch, D. A.
2001 A meta-analysis comparing buprenorphine to methadone for treatment of opiate dependence. Addiction, 96(5), 683-690.
Beck A.J., Karberg J.C., & Harrison P.M.
2002 Prison and jail inmates at midyear 2001 (No. NCJ 191702): Washington, D.C.: US Department of Justice.
Bird, A. G, Gore, S. M., Cameron, S., Ross, A. J., & Goldberg, D. J.
1995 Anonymous HIV surveillance with risk factor elicitation at Scotland's largest prison, Barlinnie. AIDS, 9(7), 801-808.
Blankenship, K. M., Bray, S. J., & Merson, M. H.
2000 Structural interventions in public health. AIDS, 14 Suppl 1, 811-21.
Brahen, L. S., Henderson, R. K., Capone, T., & Kordal, N.
1984 Naltrexone treatment in a jail work-release program. Journal of Clinical Psychiatry, 45(9 Pt 2), 49-52.
Brooke, D., Taylor, C., Gunn, J., & Maden, A.
1998 Substance misusers remanded to prison - a treatment opportunity? Addiction, 93(12), 1851-1856.
Butzin, C. A., Martin, S. S., & Inciardi, J. A.
2002 Evaluating component effects of a prison-based treatment continuum. Journal of Substance Abuse Treatment, 22(2), 63-69.
Byrne, A., & Dolan, K.
1998 Methadone treatment is widely accepted in prisons in New South Wales. British Medical Journal, 316(7146), 1744-1745.
Chaiken, J. M.
2000 Correctional population of the United States, 1997 (No. NCJ 177613). Washington, D.C.: US Department of Justice.
Chanhatasilpa, C., MacKenzie, D. L., & Hickman, L. J.
2000 The effectiveness of community-based programs for chemically dependent offenders: A review and assessment of the research. Journal of Substance Abuse Treatment, 19(4), 383-393.
Chick, J., Anton, R., Checinski, K., Croop, R., Drummond, D.C., Farmer, R., Labriola, D., Marshall, J., Moncrieff, J., Morgan, M. Y., Peters, T., & Ritson, B.
2000 A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol and Alcoholism, 35(6), 587-593.
Clark, N., Lintzeris, N., Gijsbers, A., Whelan, G., Dunlop, A., Ritter, A., & Ling, W.
2002 LAAM maintenance vs. methadone maintenance for heroin dependence. Cochrane Database of Systematic Reviews, 2, CD002210.
Claudon-Charpentier, A., Hoibian, M., Classer, P., Lalanne, H., & Pasquali, J. L.
2000 Drug-addicted prisoners: Seroprevalence of human immunodeficiency virus and hepatitis B and C virus soon after the marketing of buprenorphine. Revue de Medecine Interne, 21(6), 505-509.
Comer, S. D., Collins, E. D., Kleber, H. D., Nuwayser, E. S., Kerrigan, J. H., & Fischman, M. W.
2002 Depot naltrexone: Long-lasting antagonism of the effects of heroin in humans. Psychopharmacology (Berl), 159(4), 351-360.
Cornish, J. W., Metzger, D., Woody, G. E., Wilson, D., McLellan, A. T., Vandergrift, B., & O'Brien, C. P.
1997 Naltrexone pharmacotherapy for opioid dependent federal probationers. Journal of Substance Abuse Treatment, 14(6), 529-534.
Crowley, T. J., Wagner, J. E., Zerbe, G., & Macdonald, M.
1985 Naltrexone-induced dysphoria in former opioid addicts. American Journal of Psychiatry, 142(9), 1081-1084.
De Leon, G.
1996 Therapeutic communities: AIDS/HIV risk and harm reduction. Journal of Substance Abuse Treatment, 13(5), 411-420.
De Leon, G., Melnick, G., Kressel, D., & Jainchill, N.
1994 Circumstances, motivation, readiness, and suitability (the CMRS scales): Predicting retention in therapeutic community treatment. American Journal of Drug Alcohol Abuse, 20(4), 495-515.
Desland, M., & Batey, R.
1991 High retention rates within a prospective study of heroin users. British Journal of Addiction, 86(7), 859-865.
Dolan, K., Hall, W., & Wodak, A.
1996 Methadone maintenance reduces injecting in prison. British Medical Journal, 312(7039), 1162.
Dole, V. P., Nyswander, M. E., & Kreek, M. J.
1966 Narcotic blockade. Archives of Internal Medicine, 118(4), 304-309.
Donny, E. C., Walsh, S. L., Bigelow, G. E., Eissenberg, T., & Stitzer, M. L.
2002 High-dose methadone produces superior opioid blockade and comparable withdrawal suppression to lower doses in opioid-dependent humans. Psychopharmacology (Berl), 161(2), 202-212.
Doran, C. M., Shanahan, M., Mattick, R. P., AIi, R., White, J., & Bell, J.
2003 Buprenorphine versus methadone maintenance: A cost-effectiveness analysis. Drug and Alcohol Dependence, 71(3), 295-302.
Durand, E.
2001 Changes in high-dose buprenorphine maintenance therapy at the FleuryMerogis (France) prison since 1996. Annales de Medecine Interne (Paris), 152(Suppl 7), 9-14.
Edlin, B. R.
2002 Prevention and treatment of hepatitis C in injection drug users. Hepatology, 36(5 Suppl 1), S210-219.
Farre, M., Mas, A., Torrens, M., Moreno, V., & Cami, J.
2002 Retention rate and illicit opioid use during methadone maintenance interventions: A meta-analysis. Drug and Alcohol Dependence, 65(3), 283-290.
Farren, C. K., O'Malley, S., & Rounsaville, B.
1997 Naltrexone and opiate abuse. In S.M. Stine & T.R. Kosten (Eds.), New treatments for opiate dependence. The Guilford substance abuse series (pp. 104-123). Guilford, CT: Guilford Press.
Favrat, B., Rao, S., O'Connor, P. G., & Schottenfeld, R.
2002 A staging system to predict prognosis among methadone maintenance patients, based on admission characteristics. Substance Abuse, 23(4), 233-244.
Feeney, G. F., Young, R. M., Connor, J. P., Tucker, J., & McPherson, A.
2001 Outpatient cognitive behavioural therapy programme for alcohol dependence: Impact of naltrexone use on outcome. Australian and New Zealand Journal Psychiatry, 35(4), 443-448.
Fhima, A., Henrion, R., Lowenstein, W., & Charpak, Y.
2001 Two-year follow-up of an opioid-user cohort treated with high-dose buprenorphine (Subutex). Annales de Medecine Interne (Paris), 152(Suppl 3), IS26-36.
Fiellin, D. A., & O'Connor, P. G.
2002 Clinical practice. Office-based treatment of opioid-dependent patients. New England Journal of Medicine, 347(11), 817-823.
Fiellin, D. A., O'Connor, P. G, Chawarski, M., Pakes, J. P., Pantalon, M. V., & Schottenfeld, R. S.
2001 Methadone maintenance in primary care: a randomized controlled trial. Journal of the American Medical Association, 286(14), 1724-1731.
Fiellin, D. A., Pantalon, M. V., Pakes, J. P., O'Connor, P. G, Chawarski, M., & Schottenfeld, R. S.
2002 Treatment of heroin dependence with buprenorphine in primary care. American Journal of Drug and Alcohol Abuse, 28(2), 231-241.
Friedmann, P. D., Lemon, S. C., & Stein, M. D.
2001 Transportation and retention in outpatient drug abuse treatment programs. Journal of Substance Abuse Treatment, 21(2), 97-103.
Fudala, P. J., Jaffe, J. H., Dax, E. M., & Johnson, R. E.
1990 Use of buprenorphine in the treatment of opioid addiction. II. Physiologic and behavioral effects of daily and alternate-day administration and abrupt withdrawal. Clinical Pharmacology and Therapeutics, 47(4), 525-534.
Garfein, R., Vlahov, D., Galai, N., Doherty, M., & Nelson, K.
1996 Viral infections in short-term injection drug users: The prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. American Journal of Public Health, 86(5), 655-661.
Glaser, J. B., & Greifmger, R. B.
1993 Correctional health care: a public health opportunity. Annals of Internal Medicine, 118(2), 139-145.
Gonzalez, J. P., & Brogden, R. N.
1988 Naltrexone. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs, 35(3), 192-213.
Gowing, L., Ali, R., & White, J.
2002 Buprenorphine for the management of opioid withdrawal. Cochrane Database of Systematic Reviews, 2, CD002025.
Greenstein, R. A., Evans, B. D., McLellan, A. T., & O'Brien, C. P.
1983 Predictors of favorable outcome following naltrexone treatment. Drug and Alcohol Dependence, 12(2), 173-180.
Griffith, J. D., Killer, M. L., Knight, K., & Simpson, D.D.
1999 A cost-effectiveness analysis of in-prison therapeutic community treatment and risk. The Prison Journal, 79(3), 352-368.
Gruer, L., Wilson, P., Scott, R., Elliott, L., Macleod, J., Harden, K., Forrester, E., Hinshelwood, S., McNulty, H., & Silk, P.
1997 General practitioner centred scheme for treatment of opiate dependent drug injectors in Glasgow. British Medical Journal, 314(7096), 1730-1735.
Hagan, H., Snyder, N., Hough, E., Yu, T.J., McKeirnan, S., Boase, J., & Duchin, J. 2002 Case-reporting of acute hepatitis B and C among injection drug users. Journal of Urban Health, 79(4), 579-585.
Hammett, T. M., Harmon, M. P., & Rhodes, W.
2002 The burden of infectious disease among inmates of and releasees from US correctional facilities, 1997. American Journal of Public Health, 92(11), 1789-1794.
Harlow, C. W.
1998 Profile of Jail Inmates in 1996 (No. NCJ 164620). Washington, D.C.: US Department of Justice.
Hasson, A. L., Grella, C. E., Rawson, R., & Anglin, M. D.
1994 Case management within a methadone maintenance program. A research demonstration project for HIV risk reduction. Journal of Case Management, 3(4), 167-172.
Hickman, M., Madden, P., Henry, J., Baker, A., Wallace, C., Wakefield, J., Stimson, G, & Elliott, P.
2003 Trends in drug overdose deaths in England and Wales 1993-98: Methadone does not kill more people than heroin. Addiction, 98(4), 419-425.
Hiller, M. L., Knight, K., & Simpson, D. D.
1999 Prison-based substance abuse treatment, residential aftercare and recidivism. Addiction, 94(6), 833-842.
Hser, Y. I., Anglin, M. D., & Fletcher, B.
1998 Comparative treatment effectiveness. Effects of program modality and client drug dependence history on drug use reduction. Journal of Substance Abuse Treatment, 15(6), 513-523.
Hutchinson, S.J., Taylor, A., Gruer, E., Barr, C., Mills, C., Elliott, E., Goldberg, D.J., Scott, R., & Gilchrist, G.
2000 One-year follow-up of opiate injectors treated with oral methadone in a GP-centred programme. Addiction, 95(7), 1055-1068.
Jasinski, D. R., Pevnick, J. S., & Griffith, J. D.
1978 Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Archives of General Psychiatry, 35(4), 501-516.
Johnson, R. E., Chutuape, M. A., Strain, E. C., Walsh, S. E., Stitzer, M. E., & Bigelow, G. E.
2000 A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. New England Journal of Medicine, 343(18), 1290-1297.
Johnson, R. E., Eissenberg, T., Stitzer, M. E., Strain, E.G., Eiebson, I. A., & Bigelow, GE.
1995 Buprenorphine treatment of opioid dependence: clinical trial of daily versus alternate-day dosing. Drug and Alcohol Dependence, 40(1), 27-35.
Jones, M.
1980 Desirable features of a therapeutic community in a prison. In H. Toch (Ed.), Therapeutic Communities in Corrections. New York: Praeger.
Katz, R. I.
1999 The addiction treatment unit: A dual diagnosis program at the California Medical Facility - a descriptive report. Journal of Psychoactive Drugs, 31(1), 41-46.
Kinlock, T. W., Battjes, R. J., & Schwartz, R. P.
2002 A novel opioid maintenance program for prisoners: preliminary findings. Journal of Substance Abuse Treatment, 22(3), 141-147.
Kirchmayer, U., Davoli, M., & Verster, A.
2002 Naltrexone maintenance treatment for opioid dependence. Cochrane Database of Systematic Reviews, 2, CDOO1333.
Kirchmayer, U., Davoli, M., Verster, A. D., Amato, E., Ferri, A., & Perucci, C. A.
2002 A systematic review on the efficacy of naltrexone maintenance treatment in opioid dependence. Addiction, 97(10), 1241-1249.
Kleber, H. D.
2003 Pharmacologie treatments for heroin and cocaine dependence. American Journal of Addiction, 12 Suppl, S5-S18.
Knight, K., Simpson, D. D., & Hiller, M. E.
1999 Three-year reincarceration outcomes for in-prison therapeutic community treatment in Texas. The Prison Journal, 79(3), 337-351.
Kraft, M. K., Rothbard, A. B., Hadley, T. R., McLellan, A. T., & Asch, D. A.
1997 Are supplementary services provided during methadone maintenance really cost-effective? American Journal of Psychiatry, 154(9), 1214-1219.
Kreek, M. J., & Vocci, F. J.
2002 History and current status of opioid maintenance treatments: Blending conference session. Journal of Substance Abuse Treatment, 23(2), 93-105.
Kuo, L, Brady, J., Butler, C., Schwartz, R., Brooner, R., Vlahov, D., & Strathdee, S.A.
2003 Feasibility of referring drug users from a needle exchange program into an addiction treatment program: experience with a mobile treatment van and LAAM maintenance. Journal of Substance Abuse Treatment, 24(1), 67-74.
Langan, P. A., & Levin, D. J.
2002 Recidivism of prisoners released in 1994 (No. NCJ 193427). Washington, D.C.: U.S. Department of Justice.
Levasseur, L., Marzo, J. N., Ross, N., & Blatier, C.
2002 Frequency of re-incarcerations in the same detention center: role of substitution therapy. A preliminary retrospective analysis. Annales de Medecine Interne (Paris), 153(3 Suppl), 1S14-19.
Liebman, J., Pat Lamberti, M., & Altice, F.
2002 Effectiveness of a mobile medical van in providing screening services for STDs and HIV. Public Health Nurse, 19(5), 345-353.
Liguori, A., Morse, W. H., & Bergman, J.
1996 Respiratory effects of opioid full and partial agonists in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics, 277(1), 462-472.
Ling, W., & Smith, D.
2002 Buprenorphine: blending practice and research. Journal of Substance Abuse Treatment, 23(2), 87-92.
Liu, S. J., & Wang, R. I.
1984 Relationship of plasma level and pharmacological activity of methadone. NIDA Research Monograph, 49, 128-135.
Magura, S., Rosenblum, A., Lewis, C., & Joseph, H.
1993 The effectiveness of in-jail methadone maintenance. Journal of Drug Issues, 23(1), 75-99.
Mark, T. L., Woody, G. E., Juday, T., & Kleber, H. D.
2001 The economic costs of heroin addiction in the United States. Drug and Alcohol Dependence, 61(2), 195-206.
Marsch, L. A.
1998 The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: a meta-analysis. Addiction, 93(4), 515-532.
Martin, V., Cayla, J. A., Bolea, A., & Castilla, J.
2000 Mycobacterium tuberculosis and human immunodeficiency virus co-infection in intravenous drug users on admission to prison. International journal of Tuberculosis and Lung Disease, 4(1), 41-46.
Mattick, R. P., Breen, C., Kimber, J., & Davoli, M.
2002 Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence Cochrane Database of Systematic Reviews, 4, CD002209.
Mattick, R. P., Kimber, J., Breen, C., & Davoli, M.
2002 Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence Cochrane Database of Systematic Reviews, 4, CD002207.
McLellan, A. T., Alterman, A. I., Metzger, D. S., Grissom, G. R., Woody, G. E., Luborsky, L., O'Brien, C. P.
1994 Similarity of outcome predictors across opiate, cocaine, and alcohol treatments: Role of treatment services. Journal of Consulting and Clinical Psychology, 62(6), 1141-1158.
McLellan, A. T., Arndt, I. O., Metzger, D. S., Woody, G. E., & O'Brien, C. P.
1993 The effects of psychosocial services in substance abuse treatment. Journal of the American Medical Association, 269(15), 1953-1959.
Milby, J. B., Sims, M..K., Khuder, S., Schumacher, J. E., Muggins, N., McLellan, A.T., Woody, G, Haas, N.
1996 Psychiatric comorbidity: prevalence in methadone maintenance treatment. American Journal of Drug and Alcohol Abuse, 22(1), 95-107.
Modesto-Lowe, V.
2002 Naltrexone depot (Drug Abuse Sciences). Idrugs, 5(8), 835-838.
Mumola, C.
1999 Substance abuse and treatment, state and federal prisoners, 1997 (No. NCJ 172871). Washington, D.C.: US Department of Justice.
National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction
1998 Effective medical treatment of opiate addiction. Journal of the American Medical Association, 280(22), 1936-1943.
Neff, J. A., & Moody, D. E.
2001 Differential N-demethylation of 1-alpha-acetylmethadol (LAAM) and norLAAM by cytochrome P450s 2B6, 2C18, and 3A4. Biochemical and Biophysical Research Communications, 284(3), 751-756.
Nielsen, A. L., Scarpitti, F. R., & Inciardi, J. A.
1996 Integrating the therapeutic community and work release for drug-involved offenders. The CREST Program. Journal of Substance Abuse Treament, 13(4), 349-358.
Nurco, D. N., Hanlon, T. E., & Kinlock, T. W.
1991 Recent research on the relationship between illicit drug use and crime. Behavioral Science and the Law, 9(3), 221-242.
Obadia, Y., Perrin, V., Feroni, L, Vlahov, D., & Moatti, J. P.
2001 Injecting misuse of buprenorphine among French drug users. Addiction, 96(2), 267-272.
O'Connor, P. G, Oliveto, A. H., Shi, J. M., Triffleman, E. G, Carroll, K. M., Kosten, T. R., Rounsaville, B. J., Pakes, J. A., & Schottenfeld, R. S.
1998 A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. American Journal of Medicine, 105(2), 100-105.
Oda, Y., & Kharasch, E. D.
2001 Metabolism of levo-alpha-Acetylmethadol (LAAM) by human liver cytochrome P450: involvement of CYP3 A4 characterized by atypical kinetics with two binding sites. Journal of Pharmacology and Experimental Therapeutics, 297(1), 410-422.
Osemene, N. I., Essien, E. J., & Egbunike, I. G.
2001 HIV/AIDS behind bars: an avenue for culturally sensitive interventions. Journal of the National Medical Association, 93(12), 481-486.
Pollack, H. A., Khoshnood, K., & Altice, F. L.
1999 Health Care Delivery Strategies for Supervised Offenders. Journal of Health Care Finance, 26(1), 1-17.
Raisch, D. W., Fye, C. L., Boardman, K. D., & Sather, M. R.
2002 Opioid dependence treatment, including buprenorphine/naloxone. Annals of Pharmacotherapy, 36(2), 312-321.
Rettig, R. A., & Yarmolinsky, A.
1995 Federal regulation of methadone treatment. Washington, D.C.: Institute of Medicine.
Ritter, A.J., Lintzeris, N., Clark, N., Kutin, J. J., Bammer, G, & Panjari, M. 2002 LAAM maintenance vs methadone maintenance for heroin dependence. Cochrane Database Syst Rev(2), CD002210.
Robinson, G. M., Dukes, P. D., Robinson, B. J., Cooke, R. R., & Mahoney, G. N.
1993 The misuse of buprenorphine and a buprenorphine-naloxone combination in Wellington, New Zealand. Drug and Alcohol Dependence, 33(1), 81-86.
Roth, A., Hogan, I., & Farren, C.
1997 Naltrexone plus group therapy for the treatment of opiate-abusing healthcare professionals. Journal of Substance Abuse Treatment, 14(1), 19-22.
Rothon, D. A.
1997 Methadone in provincial prisons in British Columbia. Canadian HIV and AIDS Policy Law Newsletter, 3-4(4-1), 27-31.
Rouse, J. J.
1991 Evaluation research on prison-based drug treatment programs and some policy implications. International Journal of the Addictions, 26(1), 29-44.
Substance Abuse and Mental Health Services Administration.
2001 National Household Survey of Drug Abuse. Rockville, MD: Substance Abuse and Mental Health Services Administration.
Schuh, K. J., Walsh, S. L., Bigelow, G. E., Preston, K. E., & Stitzer, M. L.
1996 Buprenorphine, morphine and naloxone effects during ascending morphine maintenance in humans. Journal of Pharmacology and Experimental Therapeutics, 278(2), 836-846.
Sheu, M., Hogan, J., Allsworth, J., Stein, M., Vlahov, D., Schoenbaum, E. E., Schuman, P., Gardner, E., & Flanigan, T.
2002 Continuity of medical care and risk of incarceration in HIV-positive and high-risk HIV-negative women. Journal of Womens Health (Larchmt), 11(8), 743-750.
Shwartz, M., Baker, G, Mulvey, K. P., & Plough, A.
1997 Improving publicly funded substance abuse treatment: the value of case management. American Journal of Public Health, 87(10), 1659-1664.
Sibbald, B.
2002 Methadone maintenance expands inside federal prisons. Canadian Medical Association Journal, 167(10), 1154.
Skolnick, A. A.
1998a Critics denounce staffing jails and prisons with physicians convicted of misconduct. Journal of the American Medical Association, 280(16), 1391-1392.
Skolnick, A. A.
1998b Prison deaths spotlight how boards handle impaired, disciplined physicians. Journal of the American Medical Association, 280(16), 1387-1390.
Spaulding, A., Greene, C., Davidson, K., Schneidermann, M., & Rich, J.
1999 Hepatitis C in state correctional facilities. Preventive Medicine, 28(1), 92-100.
Sporer, K. A.
2003 Strategies for preventing heroin overdose. British Medical Journal, 326(7386), 442-444.
Stoller, K. B., Bigelow, G. E., Walsh, S. L., & Strain, E. C.
2001 Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology (Berl), 154(3), 230-242.
Strain, E. C., Bigelow, G. E., Liebson, I. A., & Stitzer, M. L.
1999 Moderate- vs high-dose methadone in the treatment of opioid dependence: A randomized trial. Journal of the American Medical Assocation, 281(11), 1000-1005.
Strain, E. C., Stitzer, M. L., Liebson, I. A., & Bigelow, G. E.
1996 Buprenorphine versus methadone in the treatment of opioid dependence: Self-reports, urinalysis and addiction severity index. Journal of Clinical Psychopharmacoly, 16(1), 58-67.
Thompson, A. S., Blankenship, K. M., Selwyn, P. A., Khoshnood, K., Lopez, M., Balacos, K., Altice, F. L.
1998 Evaluation of an innovative program to address the health and social service needs of drug-using women with or at risk for HIV infection. Journal of Community Health, 23(6), 419-440.
Tomasino, V., Swanson, A. J., Nolan, J., & Shuman, H. I.
2001 The Key Extended Entry Program (KEEP): A methadone treatment program for opiate-dependent inmates. Mount Sinai Journal of Medicine, 68(1), 14-20.
Tzschentke, T. M.
2002 Behavioral pharmacology of buprenorphine, with a focus on preclinical models of reward and addiction. Psychopharmacology (Berl), 161(1), 1-16.
Vormfelde, S. V., & Poser, W.
2001 Death attributed to methadone. Pharmacopsychiatry, 34(6), 217-222.
Wall, R., Rehm, J., Fischer, B., Brands, B., Gliksman, L., Stewart, J., Medved, W., & Blake, J.
2000 Social costs of untreated opioid dependence. Journal of Urban Health, 77(4), 688-722.
Walsh, S. E., Preston, K. L., Stitzer, M. E., Cone, E. J., & Bigelow, G. E.
1994 Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clinical Pharmacology and Therapeutics, 55(5), 569-580.
Washton, A. M., Gold, M. S., & Pottash, A.
1984 Successful use of naltrexone in addicted physicians and business executives. Advances in Alcohol and Substance Abuse, 4(2), 89-96.
Wexler, H. K., Melnick, G., Lowe, E., & Peters, J.
1999 Three-year reincarceration outcomes for amity in-prison therapeutic community and aftercare in California. The Prison Journal, 79(3), 321-336.
Widman, M., Platt, J. J., Eidz, V., Mathis, D. A., & Metzger, D. S.
1997 Patterns of service use and treatment involvement of methadone maintenance patients. Journal of Substance Abuse Treatment, 14(1), 29-35.
World Health Organization
1993 WHO Guidelines on HIV Infection and AIDS in Prisons. Geneva: World Health Organization.
Yoast, R., Williams, M. A., Deitchman, S. D., & Champion, H. C.
2001 Report of the Council on Scientific Affairs: Methadone maintenance and needle-exchange programs to reduce the medical and public health consequences of drug abuse. Journal of Addictive Diseases, 20(2), 15-40.
Zaric, G. S., Barnett, P. G, & Brandeau, M. E.
2000 HIV transmission and the cost-effectiveness of methadone maintenance. American Journal of Public Health, 90(7), 1100-1111.
Zule, W. A., & Desmond, D. P.
1998 Attitudes toward methadone maintenance: Implications for HIV prevention. Journal of Psychoactive Drugs, 30(1), 89-97.
Duncan Smith-Rohrberg is an M.D./Ph.D. candidate in public health at the Yale University School of Medicine and a National Institutes of Health Medical Scientist Training Program scholar. Robert D. Bruce, M.D., is an instructor at the Yale University AIDS Program at the Yale University School of Medicine. Frederick L. Altice, M.D. is an associate professor of medicine in the Yale University AIDS Program and the director of the HIV in Prisons Program at the Yale University School of Medicine.
Copyright Florida State University for and on behalf of The Florida State University Board of Trustees Spring 2004
Provided by ProQuest Information and Learning Company. All rights Reserved