A 41-year-old woman had progressive shortness of breath. Cerebrotendinous xanthomatosis was diagnosed 4 years before. An open-lung biopsy showed the simultaneous presence of cerebrotendinous xanthomatosis and pulmonary lymphangioleiomyomatosis. This is perhaps the first time the coineidental occurrenee of these two diseases is described. (CHEST 1997; 112:273-74)
Key words: cerebrotendinous xanthomatosis; lymphangioleiomyomatosis; uterine leiomyosarcoma
Abbreviations: CTX=cerebrotendinous xanthomatosis; LAM= lymphangioleiomyomatosis
The differential diagnosis of progressive dyspnea comprises many different diseases. Coincidence of diseases causing dyspnea is not unusual when at least one of the diseases is relatively common or diseases are related. However, this report presents the case of a patient suffering from two extremely rare diseases, both afflicting pulmonary function: pulmonary lymphangioleiomyomatosis (LAM) and cerebrotendinous xanthomatosis (CTX). These diseases are believed to be unrelated.
CASE REPORT
A 41-year-old woman with no history of lung disease was examined because of progressive exercise-induced dyspnea (without coughing, wheezing, pain, or fever) that had started about 5 months before admission. Her medical history mentioned a progressive spastic paresis, cognitive deterioration, and a bilateral cataract, caused by CTX, which was diagnosed 4 years before. The diagnosis of CTX was genetically confirmed (homozygote for a point mutation in exon 5 of the human sterol 27 hydroxylase gene[1]).
Results of physical examination were, apart from obesity (length, 1.63 m; weight, 80 kg), normal. With the patient at rest, arterial blood gas analysis revealed [Po.sub.2] to be 65 mm Hg; pH, 7.45; [Pco.sub.2], 32 mm Hg; bicarbonate, 22.3 mmol/L (breathing air). Results of pulmonary function tests showed mild airflow obstruction ([FEV.sub.1], 1.7 L (2.7 L predicted value), inspiratory vital capacity 3.30 L (3.33 L predicted value), and loss of diffusing capacity (CO-transfer factor, 0.65 mmol/min/kPa). During exercise of 40-W, a decrease of [Po.sub.2] to 56 mm Hg and of [Pco.sub.2] to 30 mm Hg was observed, while the alveolar-arterial oxygen pressure difference increased from 48 to 70 mm Hg. A chest radiograph revealed a homogeneous bilateral interstitial pattern of infiltration. A high-resolution CT scan showed thin-wailed cysts in all parts of both lungs. On bronchoscopy, no endobronchial abnormalities were seen. The biopsy specimens also showed no abnormalities. Analysis of BAL fluid disclosed no abnormalities. A biopsy specimen from the lingula showed on transsected lung tissue cystic structures, measuring 2 to 5 mm. Microscopically, the interstitium showed a multifocal proliferation of smooth muscle cells, along bronchioli and lymph vessels (Fig 1). In some places, nodular fascicles of smooth muscles were arranged as cystic structures. The smooth muscle differentiation was confirmed immunohistochemically. This histologic picture is consistent with the diagnosis of pulmonary LAM. Besides this, focally in the alveoli there was abundant accumulation of large foam cells and some multinucleated giant cells with cholesterol clefts (Fig 2). These findings are likely to be attributed to CTX and are not consistent with LAM.
[Figure 1 & 2 ILLUSTRATION OMITTED]
Four months later, the patient underwent a total hysterectomy because of severe hypermenorrhea. During this procedure a bilateral oophorectomy was performed by request of the physicians. Histologic examination of the uterus revealed a low-grade leiomyosarcoma.
DISCUSSION
Pulmonary LAM is an extremely rare disease afflicting women of childbearing age. Because of its rarity, literature on this disease is mostly anecdotal. Recently, two reports on larger series of patients with LAM have been published.[2,3] LAM usually presents with spontaneous pneumothorax, chylothorax, hemoptysis, or slowly progressive dyspnea. The diffuse distribution instead of a macronodular appearance, on a chest radiography and histologically, differentiates LAM from benign metastasizing leiomyoma, a disease that has much in common with LAM. In this patient, the homogeneous pattern, the presence of multiple cysts, and the absence of both mitotic figures and an invasive growing pattern of the smooth muscle cells in the lung biopsy specimen pleads against a relationship with the low-grade leiomyosarcoma of the uterus. However, it is appealing to speculate on such a relationship in this patient; a change in hormonal sensitivity may be involved in the development of not only LAM but also the uterine leiomyosarcoma (estrogen and progesterone receptors are present in uterine leiomyosarcoma tumor cells[4]).
Tuberous sclerosis involving the lungs is indistinguishable from LAM.[5] However, in this patient the neurologic picture (typical for CTX and not for tuberous sclerosis), the genetic confirmation of the presence of CTX, and the absence of extrapulmonary manifestations congruent with tuberous sclerosis rule out tuberous sclerosis as a possible diagnosis.
Therapies described for LAM consist of several modes of hormonal manipulation. Because of poor compliance with therapy, we decided to perform a bilateral oophorectomy. Recent reports show that the average survival in patients with LAM is approximately 10 years.[2,3]
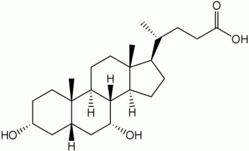
CTX is a rare (150 patients described in the medical literature) autosomal recessive lipid storage disease caused by a defect in the activity of the mitochondrial enzyme sterol 27-hydroxylase.[6] This deficiency leads to a reduced synthesis of cholic acid, whereas almost no chenodeoxycholic acid is produced. Excessive amounts of cholestanol are produced, which accumulate in many tissues.[7]
Accepted clinical characteristics of CTX are premature bilateral cataract, formation of tendon xanthomas, premature coronary heart disease, and neurologic abnormalities.[4,7] Information of lung involvement in CTX patients is sparse. None of the patients described in the medical literature had progressive dyspnea. There are a few autopsy studies. In 1937, Bogaert et al[8] found pleural granulomatous lesions containing foam cells and needle-shaped clefts. In 1968, Schimschock et al[9] found these lesions in a 58-year-old CTX patient within the lungs. They described accumulation of free fat around blood vessels and throughout the granulomatous lesions. Many hemosiderin-containing macrophages were seen. In our patient, an excess of foam ceils was present in a patchy way in the biopsy material. Many of the respiratory symptoms probably are caused by LAM, since LAM was present to a much greater extent than histologic changes due to CTX.
There does not seem to be a relationship between CTX and LAM. However, the minuscule chance that there is a coincidence in the occurrence of these two very rare diseases suggests a yet unknown connection.
REFERENCES
[1] Reshef A, Meiner V, Berginer VM, et al. Molecular genetics of cerebrotendinous xanthomatosis in Jews of north African origin. J Lipid Res 1994; 35:478-83
[2] Taylor JR, Ryu J, Colby TV, et al. Lymphangioleiomyomatosis: clinical course in 32 patients. N Engl J Med 1990; 323:1254-60
[3] Kitaichi M, Nishimura K, Itoh H, et al. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med 1995; 151:527-33
[4] Soper JT, McCarty KSJ, Hinshaw W, et al. Cytoplasmic estrogen and progesterone receptor content of uterine sarcomas. Am J Obstet Gynecol 1984; 150:342-48
[5] Stovin PG, Lum LC, Flower CD, et al. The lungs in lymphangioleiomyomatosis and in tuberous sclerosis. Thorax 1975; 30:497-509
[6] Oftebro H, Bjorkhem I, Stormer FC, et al. Cerebrotendinous xanthomatosis: defective liver mitochondrial hydroxylation of chenodeoxycholic acid precursors. J Lipid Res 1981; 22:632-40
[7] Boberg KM, Bjorkhem I. Inborn errors in bile acid biosynthesis and storage of sterols other than cholesterol. In: Scriver C, ed. The metabolic and molecular basis of inherited disease. New York: McGraw-Hill 1995; 2282-99
[8] Bogaert L, Scherer H, Epstein E. Une forme cerebrale de la cholesterinose generalisee. Paris: Masson, 1937
[9] Schimschock JR, Alvord ECJ, Swanson PD. Cerebrotendinous xanthomatosis: clinical and pathological studies. Arch Neurol 1968; 18:688-98
(*) From the Departments of Internal Medicine (Dr. Dormans), Neurology (Dr. Verrips), Pathology (Dr. Bulten), and Pulmonology (Dr. Cox), University Hospital Nijmegen, Nijmegen, The Netherlands.
Manuscript received September 16, 1996; revision accepted December 20.
Reprint requests: Dr. Dormans, Department of Internal Medicine, University of Nijmegen, P. O. Box 9101, 6500 HB Nijmegen, The Netherlands
COPYRIGHT 1997 American College of Chest Physicians
COPYRIGHT 2004 Gale Group