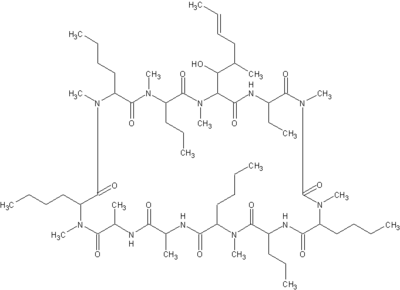
Ciclosporin
Cyclosporine, Ciclosporin (INN), or cyclosporin (former BAN), is an immunosuppressant drug. It is widely used post-allogenic organ transplant to reduce the activity of the patient's immune system and so the risk of organ rejection. It has been studied in transplants of skin, heart, kidney, lung, pancreas, bone marrow and small intestine. Cyclosporine is a cyclic nonribosomal peptide of 11 amino acids (an undecapeptide) produced by the fungus Hypocladium inflatum gams, initially isolated from a Norwegian soil sample. more...
Naming
Although the international nonproprietary name is now ciclosporin, it is still referred to as cyclosporine in most scientific journals and medical publications.
Commercialisation
The drug is marketed by Novartis under the brand names Sandimmune, the original formulation, and Neoral for the newer microemulsion formulation. Generic cyclosporine preparations have been marketed by companies such as Sangstat, Abbott Laboratories and Gengraf. Since 2002 a topical emulsion of cyclosporine for treating keratoconjunctivitis sicca has been marketed under the trade name Restasis. Annual sales of cyclosporine are around $1 billion.
Uses
The immuno-suppressive effect of Cyclosporine was discovered on January 31, 1972, by employees of Sandoz (now Novartis) in Basle, Switzerland, in a screening test on immune-suppression designed and implemented by Hartmann F. Stähelin. Cyclosporine was subsequently approved for use in 1983.
Apart from in transplant medicine, cyclosporine is also used in psoriasis and infrequently in rheumatoid arthritis and related diseases, although it is only used in severe cases. It has been investigated for use in many other autoimmune disorders. It is often taken in conjunction with corticosteroids. More recently, cyclosporine has begun to be used to help treat patients suffering from ulcerative colitis with positive results.
Cyclosporine A has been investigated as a possible neuroprotective agent in conditions such as traumatic brain injury, and has been shown in animal experiments to reduce brain damage associated with injury . Cyclosporine A blocks the formation of the mitochondrial permeability transition pore, which has been found to cause much of the damage associated with head injury and neurodegenerative diseases.
Mode of action
Cyclosporine is thought to bind to the cytosolic protein cyclophilin (immunophilin) of immunocompetent lymphocytes, especially T-lymphocytes. This complex of cyclosporin and cyclophylin inhibits calcineurin, which under normal circumstances is responsible for activating the transcription of interleukin-2. It also inhibits lymphokine production and interleukin release and therefore leads to a reduced function of effector T-cells. It does not affect cytostatic activity.
Side-effects and interactions
Treatment has a number of potentially serious side effects and has adverse interactions with a wide variety of other drugs and other materials including grapefruit, although there have been studies to improve the blood level of cyclosporine with grapefruit juice. Side effects can include gum hyperplasia, convulsions, peptic ulcers, pancreatitis, fever, vomiting, diarrhea, confusion, breathing difficulties, numbness and tingling, pruritus, high blood pressure, kidney and liver disfunction, potassium retention and possibly hyperkalemia, hepatotoxicity, nephrotoxicity, and obviously an increased vulnerability to opportunistic fungal and viral infections.
Read more at Wikipedia.org