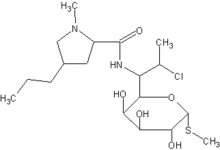
Dear Dr. Robins:
I was most interested in the article by Tschen and Jones concerning the clinical and antimicrobial results of the topical combination product containing clindamycin and benzoyl peroxide (1). Previous publications have also revealed the mixture of topical antibiotics and benzoyl peroxide diminishes the multiplication of antibiotic-resistant strains (2). Such combination therapies are hypothesized to gain their efficacy by the coupled action of two effective acne treatments. Of note, there has been a dearth of chemical data proving any synergistic activity with such a combination.
In the laboratory, I have compared the radical activity of benzoyl peroxide alone, and with various antibiotics, to determine whether there might be some synergism chemically (3). In the case of benzoyl peroxide and erythromycin, increased amounts of benzoyl peroxide derived radicals were likely formed through the interaction of benzoyl peroxide with the tertiary amine on the erythromycin molecule3. This catalysis of benzoyl peroxide radical formation may have far greater function than benzoyl peroxide and erythromycin alone in terms of the myriad oxidized intermediates that may interact with various constituents of microbial cells and the Propionibacterium acnes biofilm (4).
By using polymerization of tetra ethylene glycol dimethacrylate as the test of benzoyl peroxide radical activity, a search for other chemicals with tertiary amines or similar structures to potentiate radical formation by benzoyl peroxide can be ascertained for which a patent is pending. We did not test clindamycin in our initial study, but this may prove warranted as in vitro studies, with just parameters, are useful for comparative analysis.
Bibliography:
(1.) Tschen E, Jones T. A new treatment for acne vulgaris combining benzoyl peroxide with clindamycin. J Drugs Dermatol 2002; 1:153-7.
(2.) Warner GT, Plosker GL (Brown M, Burkhart CN, Hickman JG, Strasburger VC, West DP, contributors). Clindamycin/benzoyl peroxide gel: A review of its use in the management of acne. Am J Clin Dermatol 2002; 3:349-60.
(3.) Burkhart CN, Specht M, Neckers D. Synergistic activity of benzoyl peroxide and erythromycin. Skin Pharmacology and Applied Skin Physiology 2000; 13:292-296.
(4.) Burkhart CN, Burkhart CG. Microbiologic principle of biofilms as a major factor in pathogenesis of acne. International Journal of Dermatology (accepted 2002).
Craig N. Burkhart
Department of Immunology and Microbiology
Medical College of Ohio
5600 Monroe Street, Suite 106B
Sylvania, Ohio 43560
E-mail: Cburkhart@mco.edu
COPYRIGHT 2002 Journal of Drugs in Dermatology
COPYRIGHT 2003 Gale Group