METHOD OF PREPARATION
Note: This preparation should be done in a laminar airflow hood in a cleanroom or via isolation barrier technology by a validated aseptic compounding pharmacist using strict aseptic technique, according to USP Chapter Pharmacy Compounding, Sterile Preparations. This is a high-risk category preparation.
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Dissolve the clonidine hydrochloride in sufficient 0.9% sodium chloride injection to volume and mix well.
4. Filter through appropriate sterile 0.22-µm filters into sterile containers (syringes, vials or reservoirs).
5. Package and label.
PACKAGING
Package in tight, light-resistant containers.1
LABELING
Keep out of reach of children. Use only as directed. For professional use only.
STABILITY
If no sterility testing is done, a beyond-use date of up to 24 hours at room temperature, 3 days in the refrigerator or 45 days at -20°C can be used. If sterility testing is done, a beyond-use date of up to 6 months can be used for this preparation.1,2
USE
Clonidine injection is used in the treatment of hypertension and, more recently, for the treatment of intense, intractable pain caused by cancer and other conditions.3
QUALITY CONTROL
Quality-control assessment can include weight/volume, physical observation, pH, specific gravity, osmolality, assay, color, clarity, participate matter, sterility and pyrogenicity.4,5
DISCUSSION
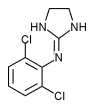
Clonidine (C^sub 9^H^sub 9^Cl^sub 2^N^sub 3^-HCl, MW 266.55, Catapres) is an imidazoline-derivative used as a central [alpha]-2-adrenergic agonist that reduces blood pressure and slows the heart rate by reducing sympathetic stimulation. It is also being used in the control of intense, intractable pain caused by cancer and other conditions and is given epidurally, intrathecally or as an intraventricular infusion. It is available as both the base and the hydrochloride salt. Clonidine hydrochloride occurs as a white, crystalline powder that has a bitter taste and is freely soluble in water and soluble in alcohol. A 5% solution in water has a pH in the range of 3.5 to 5.5; it should be stored in airtight containers.1,2
Clonidine has been extensively studied as an additive in compounded bupivacaine and lidocaine solutions, as well as in transdermal applications. For continuous epidural administration specialized techniques are required and the drug should be administered via this route only by qualified individuals familiar with the techniques of administration and patient management problems.2
Clonidine 0.1 mg/mL injection is commercially available containing clonidine hydrochloride (0.1 mg), sodium chloride (9 mg) in sufficient sterile water for injection to make 1 mL of solution. The formulation presented here is for patients requiring larger doses. The pH of the commercial solution is in the range from 5 to 7. Hydrochloric acid and/or sodium hydroxide are used to adjust the pH of the solution.6
0.9% Sodium chloride injection contains not less than 95.0% and not more than 105.0% of the labeled amount of sodium chloride in water for injection. It has a pH between 4.5 and 7.0 and contains no added antimicrobial agents. Sodium chloride solutions are chemically and physically stable. They can be sterilized by nitration or autoclaving. Aqueous sodium chloride solutions will react to form precipitates with silver, lead and mercury salts. When mixed with strong oxidizing agents, chlorine can be liberated from acidiEed sodium chloride solutions. Sodium chloride will decrease the solubility of some organic compounds; methylparaben is not as soluble in sodium chloride solutions as it is in water. Sodium chloride is soluble in water to the extent of l g in 2.8 mL water, and is slightly soluble in alcohol (1 g in 250 mL of 95% uthanol).1,7
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 27-National Formulary 22. Rockville, MD: US Pharmacopeial Convention, Inc; 2004: 486-488, 2345-2349, 2756.
2. McEvoy GK. AHFS Drug Information-2003. Bethesda, MD: American Society of Health-System Pharmacists; 2003: 1637-1645.
3. Trissel LA, Xu QA, Hassenbusch SJ. Development of clonidine hydrochloride injections for epidural and intrathecal administration. IJPC 1997; 1(4): 274-277.
4. Allen Jr LV. Standard operating procedure for particulate testing for sterile products. IJPC 1998; 2: 78.
5. Allen Jr LV. Standard operating procedure: Quality assessment for injectable solutions. IJPC 1999; 3(5): 406-407.
6. Trissel LA. Handbook on Injectable Drugs. 15th ed. Bethesda, MD: American Society of Health-System Pharmacists; 1998: 332-333.
7. Cable CG. Sodium chloride. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC: American Pharmaceutical Association; 2000: 478-481.
Copyright International Journal of Pharmaceutical Compounding May/Jun 2004
Provided by ProQuest Information and Learning Company. All rights Reserved