Cordarone
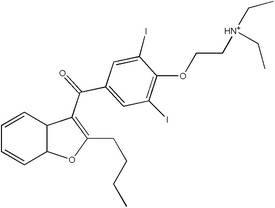
Amiodarone belongs to a class of drugs called Vaughan-Williams Class III antiarrhythmic agent. It is used in the treatment of a wide range of cardiac tachyarhthmias, including both ventricular and supraventricular (atrial) arrhythmias. The chemical name for amiodarone is 2-butyl-3-benzofuranyl 4--3,5-diiodophenyl ketone hydrochloride. more...
History
Amiodarone was initially developed in 1961 in Belgium as a treatment for angina. It was widely used throughout Europe as an anti-anginal medication, and was soon found to suppress arrhythmias.
Dr. Bramah Singh determined that amiodarone and sotalol belonged to a new class of antiarrhythmic agents (what would become the class III antiarrhythmic agents) that would prolong repolarization of the cardiac action potential. Based on this, the Argentinian physician Dr. Mauricio Rosenbaum began using amiodarone to treat his patients who suffered from supraventricular and ventricular arrhythmias, with impressive results. Based on papers written by Dr. Rosenbaum, physicians in the United States began prescribing amiodarone to their patients with potentially life-threatening arrhythmias in the late 1970s. By that time, amiodarone was commonly prescribed throughout Europe for the treatment of arrhythmias. Because amiodarone was not approved by the FDA for use in the United States at the time, physicians were forced to directly obtain amiodarone from pharmaceutical companies in Canada and Europe.
The FDA was reluctant to officially approve the use of amiodarone, since initial reports had shown increased incidence of serious pulmonary side-effects of the drug. In the mid 1980s, the European pharmaceutical companies began putting pressure on the FDA to approve amiodarone by threatening to cut the supply to the American physicians if it was not approved. In December of 1985, amiodarone was approved by the United States FDA for the treatment of arrhythmias. This makes amiodarone one of the few drugs approved by the FDA without rigorous randomized clinical trials.
Dosing
Amiodarone is available in oral and intravenous formulations. Orally, it is available under the trade names Pacerone® (produced by Upsher-Smith Laboratories, Inc.) and Cordarone® (produced by Wyeth-Ayerst Laboratories) in 200 mg and 400 mg tablets. It is also available in intravenous ampules and vials, typically in 150mg increments.
The dose of amiodarone administered is tailored to the individual and the dysrhythmia that is being treated. When administered orally, the bioavailability of amiodarone is quite variable. Absorption ranges from 22 to 95%, with better absorption when it is given with food.
Amiodarone is fat-soluble, and tends to concentrate in tissues including fat, muscle, liver, lungs, and skin. This confers a high volume of distribution (5000 liters in a 70kg adult) and a long half-life. Due to the long half-life of amiodarone, oral loading typically takes days to weeks.
Read more at Wikipedia.org