The Committee on Drugs of the American Academy of Pediatrics (AAP) has reviewed the current therapy for lead intoxication and issued guidelines for the treatment of lead exposure in children. The statement includes a review of available chelating agents and recommendations for their use. The complete statement appears in the July 1995 issue of Pediatrics.
Introduction
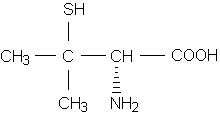
There are currently two parenteral and two oral agents used for the chelation of lead. These include dimercaprol (Bal in Oil); calcium disodium ethylenediaminetetraacetic acid (Calcium Disodium Versenate), also known as calcium EDTA; succimer (Chemet), and penicillamine (Cuprimine, Depen). Penicillamine is not approved for use in the treatment of lead poisoning.
The following recommendations have been excerpted from the AFP statement.
Recommendations
* Chelation treatment is not indicated in patients with blood lead levels of less than 25 [micro]g per dL. In these patients, environmental intervention is necessary.
* Patients with blood lead levels of 25 to 45 [micro]g per dL need aggressive environmental intervention but should not routinely receive chelation therapy, because there is no evidence that chelation avoids or reverses neurotoxicity. If blood lead levels stay in this range despite repeated environmental study and abatement, some patients may benefit from (oral) chelation therapy by enhanced lead excretion.
* Chelation therapy is indicated in patients with blood lead levels between 45 and 70 [micro]g per dL. In the absence of clinical symptoms suggesting encephalopathy (e.g., obtundation, headache and persistent vomiting), patients may be treated with succimer at 30 mg per kg per day for five days, followed by 20 mg per kg per day for 14 days. To monitor for adverse effects and to begin environmental abatement, children may need to be hospitalized for the initiation of their therapy. Children should not be discharged from the hospital until their environment has been made safe. An alternate regimen would be to use calcium EDTA as inpatient therapy (25 mg per kg per day for five days). Before chelation with either agent is started, if an abdominal radiograph shows that enteral lead is present, bowel decontamination may be considered as an adjunct to treatment.
* Patients with blood lead levels of greater than 70 [micro]g per dL or with clinical symptoms suggesting encephalopathy require inpatient chelation therapy using the most effective parenteral agents available. Lead encephalopathy is a life-threatening emergency that should be treated using contemporary standards for intensive care treatment of increased intracranial pressure, including appropriate pressure monitoring, osmotic therapy and drug therapy in addition to chelation therapy. Therapy is initiated with intramuscular dimercaprol at 25 mg per kg per day, divided into six doses. The second dose of dimercaprol is given four hours later, followed immediately by intravenous calcium EDTA at 50 mg per kg per day as a single dose, infused during several hours or as a continuous infusion. Current labeling of calcium EDTA does not support the intravenous route of administration, but clinical experience suggests that it is safe and more appropriate in children. The hemodynamic stability of these patients, as well as changes in neurologic status that may herald encephalopathy, needs to be closely monitored. Patients must be adequately hydrated to ensure renal excretion.
Therapy for all of the recommendations needs to be continued for a minimum of 72 hours. After this initial treatment, two alternatives are possible: (1) parenteral therapy with two drugs (calcium EDTA and dimercaprol) may be continued for a total of five days or (2) therapy with calcium EDTA alone may be continued for a total of five days. If dimercaprol and calcium EDTA are used for the full five days, a minimum of two days with no treatment should elapse before considering another five-day course. Before therapy is changed in patients with lead encephalopathy, parenteral chelation should be continued with both drugs until they are clinically stable.
After chelation therapy, a period of reequilibration of 10 to 14 days should be allowed before another blood lead level is obtained.
COPYRIGHT 1995 American Academy of Family Physicians
COPYRIGHT 2004 Gale Group