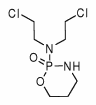
Study objectives: To prospectively examine the role of cyclophosphamide in patients with idiopathic pulmonary fibrosis that is unresponsive to or intolerant of high-dose steroid treatment.
Design: Prospective study.
Setting: Tertiary referral center.
Patients: Nineteen patients with biopsy specimen-proven usual interstitial pneumonia who failed to respond (n = 16) or experienced adverse effects (n = 3) from corticosteroid treatment (1 mg/kg/d for 3 months).
Intervention: Steroid therapy was tapered quickly, and oral cyclophosphamide, 2 mg/kg/d, was prescribed (mean duration of treatment, 6.0 [+ or -] 0.9 months).
Measurements and results: In 10 patients, response to therapy was determined by pretreatment and posttreatment clinical (dyspnea), radiographic (chest radiograph), and physiologic (pulmonary function, including exercise saturation) scores (CRP). Response was defined as a [is greater than] 10-point drop in CRP; stable as [+ or -] 10-point change in CRP; and nonresponders as [is greater than] 10-point rise in CRP. In nine patients, physiologic criteria were used to assess response; significant changes in pulmonary function were defined as follows: total lung capacity, [+ or -] 10% of baseline value; FVC, [+ or -] 10% of baseline value, diffusion capacity of the lung for carbon monoxide, [+ or -] 20% of baseline value; and resting pulse oximetry, [+ or -] 4% of baseline value. Patients who died while receiving or shortly after discontinuing cyclophosphamide were classified as nonresponders (n = 2). Among 19 patients treated with cyclophosphamide, only 1 patient demonstrated sustained response; 7 patients remained stable and 11 deteriorated while receiving the drug. Toxicity associated with cyclophosphamide was substantial; more than two thirds of the patients developed drug-related adverse effects, and almost half discontinued the drug prematurely due to side effects. In the remaining patients, cyclophosphamide therapy was discontinued due to lack of improvement or progressive deterioration.
Conclusions: Cyclophosphamide therapy is of limited efficacy in patients with idiopathic pulmonary fibrosis who fail to respond or who experience adverse effects from corticosteroid treatment, and adverse effects often complicate its use.
(CHEST 2000; 117:1619-1626)
Key words: corticosteroids; cyclophosphamide; pulmonary fibrosis; therapy; treatment
Abbreviations: CRP = clinical; radiographic; and physiologic; CTD = connective tissue disease; DECO = diffusion capacity of the lung for carbon monoxide; HRCT = high-resolution CT; HRCT-alv = high-resolution CT ground-glass opacity; HRCT-fib = high-resolution CT reticular opacity; IPF = idiopathic pulmonary fibrosis; Sp[O.sub.2] = pulse oximetry; TLC = total lung capacity; UIP = usual interstitial pneumonia
A Prospective Study in Patients Who Failed To Respond to Corticosteroids
Idiopathic pulmonary fibrosis (IPF) is an inflammatory interstitial lung disease of unknown origin. Gradual deterioration, progressing to end-stage respiratory insufficiency or death within 3 to 8 years from the onset of symptoms, is characteristic.[1-5] Corticosteroids are the mainstay of therapy, but [is less than] 30% of patients show objective response.[2,5-7] Immunosuppressive agents or cytotoxic agents (azathioprine or cyclophosphamide) have been recommended for steroid nonresponders, for patients experiencing serious adverse effects from corticosteroids, and for patients at high risk for corticosteroid complications.[6,8-11] The reported use of cyclophosphamide is limited to a few anecdotal reports and two controlled studies.[1,6,8,12-22] Despite the lack of convincing data supporting its use in patients with IPF unresponsive or intolerant to corticosteroid therapy, cyclophosphamide continues to be frequently prescribed in this group of patients.[23] Our experience with the use of this agent in IPF has been disappointing. We report on 19 patients with IPF who failed to respond or experienced adverse effects from corticosteroids and were prospectively treated with oral cyclophosphamide, 2 mg/kg/d; responses were marginal, and toxicities were significant.
MATERIALS AND METHODS
Patient Recruitment
All patients with progressive IPF who were referred to the University of Michigan for prospective enrollment in the Specialized Center of Research protocol studying IPF were considered. Suspicion of IPF was based on symptoms, physiologic abnormalities, or radiographic findings. None of the patients had undergone previous open lung biopsy or had received therapy. Patients were excluded if they were found to have a disease other than IPF during the enrollment workup. Disorders that excluded patients include the following: connective tissue disease (CTD), pneumoconiosis, sarcoidosis, cancer, lymphoma, eosinophilic granuloma, hypersensitivity pneumonitis, respiratory bronchiolitis, and lymphangioleiomyomatosis. Additionally, patients were excluded if they were unable or unwilling to undergo open or video-assisted thoracoscopic lung biopsy.
BAL
The technique used for BAL has been previously described.[24] Informed consent was obtained from all patients. The procedure was performed using an Olympus fiberoptic bronchoscope (Olympus Corporation of America; New Hyde Park, NY) after premedication with atropine and meperidine hydrochloride, and under local anesthesia with lidocaine. The tip of the bronchoscope was wedged into the right middle lobe, and 60-mL aliquots of normal saline solution were introduced and aspirated. The total fluid introduced ranged from 120 to 300 mL, and approximately one third of the introduced fluid was retrieved. The aspirated fluid was collected in a sterile container and transported on ice immediately to the laboratory. Cells in the fluid were collected by low-speed centrifugation; for morphologic examination, slide preparations were made according to techniques previously described.[24] Differential cell counts were made from total counts of 500 cells. Macrophages, lymphocytes, neutrophils, and eosinophils were identified and counted.
Physiologic Assessment
Physiologic assessment was performed before surgical (open or thoracoscopic) lung biopsy and initiation of therapy, at 3 months following treatment with corticosteroids, and after 6 months (or at the time of discontinuation) of treatment with cyclophosphamide. Pulmonary function tests, including spirometry, lung volumes, and diffusion capacity of the lung for carbon monoxide (DLCO), were performed on the same day but before cardiopulmonary exercise testing. All spirometric studies were performed using a calibrated pneumotachograph (Medical Graphics; St. Paul, MN) and values were expressed as a percent of the predicted values published by Morris et al.[25] Lung volumes were measured in a whole-body plethysmograph, and data were expressed as a percent of the predicted values published by Goldman and Becklake.[26] DLCO was corrected for measured hemoglobin and expressed as a percent of the predicted values published by Crapo and Morris.[27] Cardiopulmonary exercise tests were performed on an electronically-braked, calibrated cycle ergometer (Warren E. Collins; Braintree, MA). Workload was increased by 20 W/min until maximal symptom-limited exercise was achieved. Expired gases and ventilation were measured using a calibrated metabolic cart (2001 System; Medical Graphics; or Collins CPXII; Warren E. Collins). Arterial blood gases were obtained at rest and every 2 min during exercise via an indwelling radial artery catheter. The alveolar-arterial oxygen pressure difference was calculated for each sample by the alveolar gas equation, using the measured respiratory quotient during the same time point of exercise. Oxygen saturation was measured by co-oximetry of the blood gas sample. Twelve-lead ECGs and noninvasive measurements of BP were recorded every minute of exercise.
Clinical, Radiographic, and Physiologic Scoring System
Clinical severity was assessed for each patient using a previously developed clinical, radiographic, and physiologic (CRP) composite score for IPF.[28] The total CRP score ranges from 0 to 100 points (100 being the most severe disease), based on the following seven variables: level of dyspnea, 0 to 20 points; chest radiograph, 0 to 10 points; spirometry (FVC, 0 to 12 points; [FEV.sub.1], 0 to 3 points); lung volume, 0 to 10 points; DLCO (corrected for alveolar volume), 0 to 5 points; resting alveolar-arterial oxygen pressure difference, 0 to 10 points; and oxygen saturation corrected for maximal achieved oxygen consumption, 0 to 30 points. CRP scores were obtained at study entry, at 3 months of corticosteroid therapy, and at 6 months (or at the time of discontinuation) of cyclophosphamide treatment.
High-Resolution CT Protocol
All high-resolution CTs (HRCTs) were performed with 1.0- or 1.5-mm thick sections taken at 1-cm intervals throughout the entire thorax, and were reconstructed using a high-spatial-frequency algorithm. No IV contrast was administered. The scans were performed using a General Electric Advantage CT/T scanner (G.E. Medical Systems; Milwaukee, WI). Four experienced thoracic radiologists independently evaluated all HRCTs, as previously reported.[29] All HRCTs were obtained 1 to 4 weeks before open lung biopsy and, as such, prior to commencement of corticosteroid therapy, and again prior to the initiation of cyclophosphamide therapy. The radiologists scored ground-glass opacity and reticular opacity on a scale of 0 to 5, as previously reported.[29] For the purpose of analysis, each lobe was scored by the interpreters, and the mean of all lobes was incorporated into an HRCT ground-glass opacity score (HRCT-fib), an HRCT reticular opacity score (HRCT-alv), and a total score for each patient.
Biopsy Technique
All patients underwent bronchoscopy with BAL and transbronchial biopsies before surgical lung biopsy. Patients were referred for surgical lung biopsy when the results of transbronchial biopsy did not reveal a clear-cut alternative diagnosis. Surgical lung biopsy was performed by formal thoracotomy or video-assisted thoracoscopy. Similar-sized biopsy specimens were obtained by either technique. Biopsy specimens were obtained from all three lobes on the right side, or from the upper and lower lobe on the left with exclusion of the lingula.
Therapy
All patients were treated with prednisone, 1 mg/kg/d, for 3 months after confirmatory surgical lung biopsy and baseline studies were completed. Changes in the CRP score at 3 months were used to assess therapeutic response. Responders (a [is greater than] 10-point drop in CRP score), stable individuals (a [+ or -] 10-point change in CRP score), or nonresponders to prednisone (a [is greater than] 10-point increase in CRP score or death) were identified. Only patients classified as stable, nonresponders, and responders requiring discontinuation of prednisone due to side effects were included for the study. In all patients, corticosteroids were rapidly tapered and discontinued within 4 weeks; none of the patients experienced rapid deterioration or relapse during tapering. Patients were prescribed oral cyclophosphamide, 2 mg/kg/d, for a 6-month trial of therapy.
Response to Cyclophosphamide Therapy
Changes in the CRP score at 6 months (or at the time of discontinuation of cyclophosphamide therapy) were used to assess therapeutic response (mean duration of cyclophosphamide therapy, 6.0 [+ or -] 0.9 months). In those patients in whom CRP data were unavailable because they were unable to complete an exercise protocol (n = 9), physiologic criteria were used to assess response to treatment; significant changes in pulmonary function were defined as follows: total lung capacity (TLC), [+ or -] 10% of baseline value; FVC, [+ or -] 10% of baseline value; DLCO, + 20% of baseline value; or resting pulse oximetry (Sp[O.sub.2]), [+ or -] 4% of baseline value as used in prior studies.[6-9,12,20,24,30-32] Response was defined by a significant improvement in one or more lung function test results if it was sustained at 1 year. Nonresponders were defined as those patients who had decrements of similar magnitude in one or more lung function test results, or those who died while receiving or shortly after discontinuing cyclophosphamide therapy. Follow-up concluded with patient death or last evaluation (no patient was unavailable for follow-up).
Statistical Analysis
Those individuals remaining in stable condition were grouped and compared to those not responding to cyclophosphamide therapy using a Mann-Whitney test. Clinical features, physiologic data, and radiographic data were compared in this way. In a similar analysis, those individuals surviving were compared to those dying during long-term follow-up. There was agreement between response status as decided by CRP score and physiologic criteria using McNemar's test ([Kappa] = 0.57; p = 0.57). In all analyses, a p value [is less than] 0.05 was considered significant.
RESULTS
Patient Characteristics
Nineteen patients formed the study group, including 8 men and 11 women, with a mean age of 55.5 [+ or -] 2.7 years and 3.5 [+ or -] 1 years of symptoms prior to initiation of cyclophosphamide treatment. The histologic picture on open lung biopsy specimen demonstrated the typical, temporally heterogeneous appearance of usual interstitial pneumonia (UIP) in all patients.[33] Fourteen patients were active or ex-smokers, while 5 patients were nonsmokers. Table 1 enumerates the mean CRP score, as well as individual pulmonary function parameters and HRCT scores for the group before steroid therapy and after steroid therapy but before initiation of cyclophosphamide therapy.
Table 1--Baseline Parameters Before Steroid Therapy and After 3 Months of Steroid Therapy but Prior to Cyclophosphamide Therapy(*)
(*) Data are presented as mean [+ or -] SE.
Response to Cyclophosphamide
Figure 1 illustrates the response to cyclophosphamide therapy, and Table 2 enumerates descriptive, pulmonary function, HRCT, and BAL findings in the patients grouped by response to cyclophosphamide therapy.
[Figure 1 ILLUSTRATION OMITTED]
Table 2--Comparison of Initial Evaluation Parameters Measured After a Corticosteroid Trial but Before Institution of Cyclophosphamide Therapy Grouped by the Subsequent Response to Cyclophosphamide(*)
(*) Data are presented as mean [+ or -] SE unless otherwise indicated. Onset = duration of symptoms; NS = not significant; BAL %LM = BAL percent lymphocytes.
([dagger]) Stable vs nonresponder.
Only one patient (5.3%) demonstrated significant improvement in CRP scores at 6 months of treatment with oral cyclophosphamide (the total duration of therapy for this patient was 1.5 years). Improvement in lung function was maintained at last follow-up visit (1.8 years after cyclophosphamide treatment ended). As illustrated in Figure 1, this patient had failed to respond to an initial 3-month trial of high-dose corticosteroids therapy.
Seven patients (37%) remained in stable condition during cyclophosphamide therapy (mean duration of therapy, 5.4 [+ or -] 0.7 months; range, 2.4 to 8 months). In these patients, response to cyclophosphamide therapy was assessed by CRP in four patients and by lung function in three patients. When lung function criteria were applied to patients with available CRP scores, all remained in the stable group. Interestingly, most of these patients remained in stable condition, and one patient improved on long-term follow-up even after treatment was discontinued. Among the six patients in stable condition in whom lung function was available after cyclophosphamide was stopped (2.9 [+ or -] 0.9 years after cyclophosphamide treatment ended; range, 41 days to 5.4 years), four patients remained in stable condition (three patients were receiving no specific treatment, and one patient was receiving a low-dose prednisone/ azathioprine combination), one patient improved (a 14% increase in TLC), and one patient deteriorated (a 10% decrement in TLC). When compared to nonresponders, the patients in stable condition exhibited a trend toward a lower HRCT-fib score before initiation of cyclophosphamide therapy (Table 2).
Eleven patients (59%) were classified as nonresponders to cyclophosphamide (mean duration of therapy, 5.3 [+ or -] 0.9 months; range, 1 to 12.5 months). In these patients, the response to cyclophosphamide therapy was assessed by CRP in five patients and by lung function in four patients. Two patients died of respiratory failure while receiving or shortly after discontinuing cyclophosphamide and were classified as nonresponders (one patient died during therapy, and one patient died 3 months after discontinuing cyclophosphamide). When lung function criteria were applied to patients with available CRP scores, two patients would have been classified as being in stable condition.
Long-term Survival
At the end of the follow-up period (4.2 [+ or -] 0.4 years), seven patients died (respiratory failure [n = 6] and complications of small cell lung cancer [n = 1]). All were cyclophosphamide nonresponders. As shown in Table 3, the patients who died (n = 7) exhibited a higher pretherapy CRP score, a higher HRCT-fib score, and a higher level of dyspnea, and a trend toward a lower DLCO when compared to those who remained alive (n = 12). These data suggest that patients who died had more severe disease and increased lung fibrosis at the time of initiation of therapy compared to those who lived.
Table 3--Comparison of Initial Evaluation Parameters Measured After a Corticosteroid Trial but Before Institution of Cyclophosphamide Therapy Grouped by Survival(*)
(*) Data are presented as mean [+ or -] SE unless otherwise indicated. LOD = level of dyspnea; BAL %N = BAL percent neutrophils; see Table 2 for abbreviations.
([dagger]) Dead vs alive.
Differential Counts of Cells in BAL Fluid
As shown in Table 2, when compared to nonresponders, the group of patients remaining in stable condition during cyclophosphamide therapy exhibited a higher bronchoalveolar lymphocyte percentage at initiation of cyclophosphamide treatment (12.4 [+ or -] 6% vs 2.5 [+ or -] 0.7%; p = 0.04). No statistical differences between the groups were noted in percentage of macrophages (80.8 [+ or -] 4.8% vs 81.9 [+ or -] 4.7%; p = 0.87), neutrophils (5.4 [+ or -] 2.4% vs 12.8 [+ or -] 4.0%; p = 0.2), or eosinophils (1.5 [+ or -] 0.8% vs 12.1 [+ or -] 9.3%; p = 0.4) before cyclophosphamide therapy was started. The lack of statistical significance may have reflected the small sample size. As shown in Table 3, those patients who died vs those who remained alive exhibited a higher percentage of neutrophils at initiation of cyclophosphamide treatment (17.2 [+ or -] 5.8% vs 5.8 [+ or -] 1.9%; p = 0.04). No statistical differences between the groups were noted in the percentage of macrophages (76.6 [+ or -] 6.9% vs 84.4 [+ or -] 3.4%; p = 0.27), lymphocytes (2.4 [+ or -] 0.7% vs 8.5 [+ or -] 3.8%; p = 0.2), or eosinophils (3.8 [+ or -] 3.2% vs 10.7 [+ or -] 9.3%; p = 0.6) before cyclophosphamide therapy.
Toxicity
Thirteen patients (68%) suffered adverse effects attributable to cyclophosphamide, requiring discontinuation of therapy in 9 patients (47%). These are enumerated in Table 4. In three patients with hematologic toxicity, the reduced neutrophil and platelet counts returned to normal once the dose was reduced. In the seven patients experiencing GI toxicity (nausea, vomiting, anorexia/weight loss, or diarrhea), therapy was discontinued because of these adverse effects. Skin manifestations included skin hyperpigmentation in one patient and a morbiliform rash in one patient. None of the adverse effects were life threatening. However, a decision to discontinue therapy was made on the basis of no clinical or physiologic improvement while receiving cyclophosphamide therapy, and the desires of the patients not to continue therapy.
Table 4--Toxicity Associated With Cyclophosphamide Therapy
DISCUSSION
IPF remains a difficult therapeutic dilemma with a limited response to immunosuppressive therapy. Corticosteroids are the mainstay of therapy, but [is less than] 30% of patients show objective response.[2-6] Cyclophosphamide is commonly used in steroid nonresponders, in patients experiencing serious adverse effects from corticosteroids, and in patients at high risk for corticosteroid complications.[6,8,9] However, few studies have critically analyzed the efficacy of cyclophosphamide in IPF. We tested the hypothesis that cyclophosphamide may be efficacious in patients with UIP refractory to or intolerant of corticosteroids. Several important findings emerge from this study: (1) cyclophosphamide is of marginal efficacy in improving pulmonary function in patients with UIP who fail to respond or do not tolerate corticosteroids; (2) most patients who remain in stable condition while receiving cyclophosphamide maintain stability on long-term follow-up even after treatment has been discontinued; (3) cyclophosphamide therapy is frequently complicated by side effects; and (4) more severe disease and increased lung fibrosis are associated with worse prognosis, and identify patients unlikely to benefit from cyclophosphamide therapy.
Our prospective study evaluated oral cyclophosphamide therapy in a cohort of 19 patients with biopsy specimen-proven UIP who either failed to respond, remained in stable condition, or developed adverse effects from corticosteroids. Unfortunately, a sustained response to cyclophosphamide therapy was evident in only 1 patient; 7 patients remained in stable condition and the conditions of 11 patients deteriorated while receiving cyclophosphamide. The reported use of cyclophosphamide is limited to a few anecdotal reports and two controlled studies.[1,4,6-8,12-22,30,34,35]. Unfortunately, from these disparate studies, the efficacy of cyclophosphamide is difficult to assess for the following reasons: the uncontrolled, retrospective, or anecdotal nature of the study[6,7,12,14,16-18,20-22,30,35]; the unbalanced disease severity and substantial crossover between groups[8]; the varying diagnostic criteria without consistent use of lung biopsy[4,6,7,11,12,17,18,29]; the inclusion of patients with CTD who may exhibit better response to cytotoxic treatment[6,14-16,30]; the variable treatment regimens and follow-up[14,15,17,20,21,30]; the concurrent use of corticosteroids[8,12,14-16,18,20-22,30]; and the variable criteria used to assess response to therapy.[6,8,12,15,16,19-22,30] In contrast to previous investigations, all of our patients had histologic diagnosis of UIP and were prospectively treated with high-dose corticosteroids followed by cyclophosphamide alone. Patients with CTD and other interstitial lung diseases were excluded. In addition, objective response to cyclophosphamide was determined by rigorous pulmonary function criteria. Because the utility of pulmonary function tests to predict natural history, prognosis, and histology in IPF is limited, and a lack of consensus with regard to the variables studied exists in the literature,[4,8,12,32] we utilized the CRP scoring system because it has been shown to improve the correlation with histologic abnormalities in IPF.[28] Interestingly, although no statistical difference was evident when response to treatment was determined by CRP or by pulmonary function criteria for the group as a whole, there was a tendency to overestimate response to treatment when pulmonary function parameters were used (two nonresponder patients by CRP score would have been classified as being in stable condition by physiologic criteria); this likely contributes to the more favorable outcomes reported by other investigators.
An additional, important finding of our study is that most patients who were in stable condition while receiving cyclophosphamide therapy remained so on long-term follow-up even after treatment had been discontinued (the condition of only one patient worsened). Previous studies have reported the presence of a similar group of patients who remain in stable condition after discontinuation of therapy,[8,20,30] suggesting that cyclophosphamide may not alter the natural history of the lung disease in patients who remain in stable condition after a course of treatment. These observations cast doubt on the need to continue long-term cyclophosphamide therapy in patients with UIP who do not show a convincing response to this agent. Patients with UIP who remain in stable condition after 6 months of cyclophosphamide therapy could be advised that present therapy is unlikely to be of benefit; discontinuation of therapy in such patients would avoid potential adverse effects and reduce costs.
Toxicities associated with cyclophosphamide were frequent in our patients. More than two thirds of the patients developed drug-related adverse effects, and almost half of the patients discontinued the drug prematurely due to side effects. All seven patients with GI toxicity eventually discontinued therapy. We believe that toxicities associated with cyclophosphamide (particularly nausea and other GI adverse effects) are likely to be significantly underestimated in retrospective studies. Prospective studies employing oral cyclophosphamide, 1 to 2 mg/kg/d, combined with corticosteroids as therapy for Wegener's granulomatosis have also shown a high rate of adverse effects ([is greater than] 60%).[36-39] We did not observe life-threatening complications or malignancies in any of our patients. Other investigators have reported more serious side effects with the use cyclophosphamide with doses similar to ours, particularly when the duration of treatment has been more prolonged.[8,30,36-38,40] Enthusiasm for use of cyclophosphamide for corticosteroid-recalcitrant IPF should be tempered by potentially serious toxicities. Discriminating the patients most likely to respond to therapy is warranted to avoid unnecessarily long exposure and potential side effects.
Unfortunately, in our study, the condition of only one patient improved with cyclophosphamide therapy. Thus, no valid statistical conclusions can be generated with regard to pretreatment features associated with response to therapy. Previous studies of IPF have cited pretreatment variables associated with improved prognosis and a higher rate or response to corticosteroids, including the following: younger age, shorter duration of symptoms, female sex, a high cellularity with less fibrosis on biopsy specimens, and a high lymphocyte count in BAL fluid.[2,6,24,41] Further, in a prospective study, we demonstrated that higher pretreatment HRCT ground-glass opacities were associated with a higher likelihood of response to corticosteroids.[2] Interestingly, in our present study, the only patient who responded to cyclophosphamide was female, younger (when compared to the mean age of the other groups), had a shorter duration of symptoms (compared to nonresponders), and had a higher HRCT-alv score at initiation of therapy than the other groups. A previous report associated response to cyclophosphamide therapy with an improvement in FVC following corticosteroid therapy[12]; other retrospective studies noted a better response to cyclophosphamide therapy in patients with increased eosinophils or neutrophils in BAL fluid.[6,24] In contrast, the FVC of our responder deteriorated (from 2.42 to 1.97 L) after 3 months of corticosteroid therapy, and BAL eosinophil counts were similar among the groups. Furthermore, a higher pretreatment BAL neutrophil percentage was observed in patients who died compared to those who were alive at the end of the study. Clearly, additional studies are needed to characterize pretreatment features associated with response to cyclophosphamide.
An additional finding of our study is that all patients who died at the end of the study period failed to respond to cyclophosphamide therapy. These patients had higher pretherapy CRP score, a higher HRCT-fib score, higher levels of dyspnea, and a trend toward a lower DLCO when compared to those who were alive at the end of the study. These data suggest that these patients had more severe disease and increased lung fibrosis at the time of initiation of treatment and thus were less likely to respond to treatment. We have demonstrated that a HRCT-fib score [is greater than] 2 is highly predictive of greater mortality in patients with IPF.[2] In agreement with these findings, the mean HRCT-fib score in the group of patients who died in the current study was [is greater than] 2. We believe that a higher pretreatment HRCT-fib score may identify a group of patients who have a worse prognosis and are unlikely to respond to therapy. Withholding cyclophosphamide therapy in such patients would avoid unnecessary exposure and potential side effects.
In summary, this prospective study of patients with open biopsy specimen-proven IPF has generated several important findings: (1) cyclophosphamide is of marginal efficacy in improving pulmonary function in patients with UIP who fail to respond to or do not tolerate corticosteroids; (2) most patients who remain in stable condition when treated with cyclophosphamide remain so once the agent is stopped; (3) cyclophosphamide therapy is frequently complicated by side effects; and (4) more severe disease and increased fibrosis, as noted by a higher pretreatment HRCT-fib score, are associated with a worse prognosis and identify patients unlikely to benefit from cyclophosphamide therapy.
REFERENCES
[1] Agusti C, Xaubet Z, Roca J, et al. Interstitial pulmonary fibrosis with and without associated collagen vascular disease: results of a two year follow up. Thorax 1992; 47:1035-1040
[2] Gay SE, Kazerooni EA, Toews GB, et al. Idiopathic pulmonary fibrosis: predicting response to therapy and survival. Am J Respir Crit Care Med 1998; 157:1063-1072
[3] Schwartz DA, Helmers RA, Galvin JR, et al. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1994; 149:450-454
[4] Schwartz D, vanFossen D, Davis C, et al. Determinants of progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1994; 149:444-449
[5] Mapel DW, Samet JM, Coultas DB. Corticosteroids and the treatment of idiopathic pulmonary fibrosis: past, present, and future. Chest 1996; 110:1058-1067
[6] Rudd RM, Haslam PL, Turner-Warwick M. Cryptogenic fibrosing alveolitis: relationships of pulmonary physiology and bronchoalveolar lavage to response to treatment and prognosis. Am Rev Respir Dis 1981; 124:1-8
[7] Turner-Warwick M, Burrows B, Johnson A. Cryptogenic fibrosing alveolitis: response to corticosteroid therapy and its effect on survival. Thorax 1980; 35:593-599
[8] Johnson MA, Kwan S, Snell NJC, et al. Randomized controlled trial comparing prednisolone alone with cyclophosphamide and low dose prednisolone in combination in cryptogenic fibrosing alveolitis. Thorax 1989; 44:280-288
[9] Raghu G, DePaso WJ, Cain K, et al. Azathioprine combined with prednisone in the treatment of idiopathic pulmonary fibrosis: a prospective double-blind, randomized, placebo-controlled clinical trial. Am Rev Respir Dis 1991; 144:291-296
[10] King TE Jr. Idiopathic pulmonary fibrosis. In: Schwarz M, King T Jr., eds. Interstitial lung disease. Hamilton, OR: B.C. Decker; 1998;597-644
[11] Reynolds HY. Diagnostic and management strategies for diffuse interstitial lung disease. Chest 1998; 113:192-202
[12] van Oortegem K, Wallaert B, Marquette CH, et al. Determinants of response to immunosuppressive therapy in idiopathic pulmonary fibrosis. Eur Respir J 1994; 7:1950-1957
[13] Meier-Sydow J, Weiss SM, Buhl R, et al. Idiopathic pulmonary fibrosis: current clinical concepts and challenges in management. Semin Respir Crit Care Med 1994; 15:77-96
[14] Brown C, Turner-Warwick M. The treatment of cryptogenic fibrosing alveolitis with immunosuppressant drugs. Q J Med 1971; 158:289-302
[15] Turner-Warwick M, Haslam P. The value of serial bronchoalveolar lavages in assessing the clinical progress of patients with cryptogenic fibrosing alveolitis. Am Rev Respir Dis 1987; 135:26-34
[16] Weese W, Levine B, Kazemi H. Interstitial lung disease resistant to corticosteroid therapy: report of three cases treated with azathioprine or cyclophosphamide. Chest 1975; 67:57-60
[17] Meuret G, Fueter R, Gloor F. Early stage of fulminant idiopathic pulmonary fibrosis cured by intense combination therapy using cyclophosphamide, vincristine, and prednisone. Respiration 1978; 36:228-233
[18] Flusser G, Gurman G, Zirkin H, et al. Desquamative interstitial pneumonitis causing acute respiratory failure, responsive only to immunosuppressants. Respiration 1991; 58:324-326
[19] O'Donnell K, Keogh B, Cantin A, et al. Pharmacologic suppression of the neutrophil component of the alveolitis in idiopathic pulmonary fibrosis. Am Rev Respir Dis 1987; 136:288-293
[20] Baughman R, Lower E. Use of intermittent, intravenous cyclophosphamide for idiopathic pulmonary fibrosis. Chest 1992; 102:1090-1094
[21] Dayton C, Schwartz D, Helmers R, et al. Outcome of subjects with idiopathic pulmonary fibrosis who fail corticosteroid therapy: implications for further studies. Chest 1993; 103: 69-73
[22] Kolb M, Kirschner J, Riedel W, et al. Cyclophosphamide pulse therapy in idiopathic pulmonary fibrosis. Eur Respir J 1998; 12:1409-1414
[23] Johnston IDA, Prescott BJ, Chalmers JC, et al. British Thoracic Society study of cryptogenic fibrosing alveolitis: current presentation and initial management. Thorax 1997; 52:38-44
[24] Haslam P, Turton C, Lukoszek A, et al. Bronchoalveolar lavage fluid cell counts in cryptogenic fibrosing alveolitis and their relation to therapy. Thorax 1980; 35:328-329
[25] Morris J, Koski A, Johnson L. Spirometric standards for healthy non-smoking adults. Am Rev Respir Dis 1971; 103: 57-67
[26] Goldman H, Becklake M. Respiratory function tests: normal values at median altitudes and the prediction of normal results. Am Rev Tuberc 1959; 79:457-467
[27] Crapo R, Morris A. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis 1981; 123:185-189
[28] Watters LC, King TE, Schwarz MI, et al. A clinical, radiographic, and physiologic scoring system for the longitudinal assessment of patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis 1986; 133:97-103
[29] Kazerooni EA, Martinez FJ, Flint A, et al. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol 1997; 169: 977-983
[30] Eliasson O, Cole S, Degraff A. Adverse effects of cyclophosphamide in idiopathic pulmonary fibrosis. Conn Med 1985; 49:286-289
[31] Hanson D, Winterbauer RH, Kirtland SH, et al. Changes in pulmonary function test results after 1 year of therapy as predictors of survival in patients with idiopathic pulmonary fibrosis. Chest 1995; 108:305-310
[32] Erbes R, Schaberg T, Loddenkemper R. Lung function tests in patients with idiopathic pulmonary fibrosis: are they helpful for predicting outcome? Chest 1997; 111:51-57
[33] Flint A, Martinez FJ, Young ML, et al. Influence of sample number and biopsy site on the histologic diagnosis of diffuse lung disease. Ann Thorac Surg 1995; 60:1605-1608
[34] Hubbard R, Johnston I, Britton J. Survival in patients with cryptogenic fibrosing alveolitis: a population-based cohort study. Chest 1998; 113:396-400
[35] Terriff B, Kwan S, Chan-Yeung M, et al. Fibrosing alveolitis: chest radiography and CT as predictors of clinical and functional impairment at follow-up in 26 patients. Radiology 1992; 184:445-449
[36] Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener's granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488-498
[37] Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener's granulomatosis. Ann Intern Med 1996; 124:477-484
[38] Lynch J III, McCune J. Immunosuppressive and cytotoxic pharmacotherapy for pulmonary disorders. Am J Respir Crit Care Med 1997; 155:395-420
[39] Guillevin L, Cordier JF, Lhote F, et al. A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized Wegener's granulomatosis. Arthritis Rheum 1997; 40:2187-2198
[40] Radis CD, Kahl LE, Baker GL, et al. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis: a 20 year follow-up study. Arthritis Rheum 1995; 38:1120-1127
[41] Winterbauer RH, Hammar SP, Hallman KO, et al. Diffuse interstitial pneumonitis: clinicopathologic correlations in 20 patients treated with prednisone/azathioprine. Am J Med 1978; 65:661-672
David A. Zisman, MD; Joseph P. Lynch III, MD, FCCP; Galen B. Toews, MD, FCCP; Ella A. Kazerooni, MD, FCCP; Andrew Flint, MD, FCCP; and Fernando J. Martinez, MD, FCCP
(*) From the Department of Internal Medicine, Division of Pulmonary and Critical Care Medicine (Drs. Zisman, Lynch, Toews, and Martinez), and the Departments of Radiology (Dr. Kazerooni) and Pathology (Dr. Flint), University of Michigan, Ann Arbor, MI. Supported in part by National Institutes of Health NHLBI Grant P50HLA6487, NIH/NCRR 3 MO1 RR00042-33S3, and NIH/ NIA P60 AG08808-06.
Manuscript received June 14, 1999; revision accepted January 26, 2000.
Correspondence to: Fernando J. Martinez, MD, FCCP, Associate Professor of Internal Medicine, Division of Pulmonary and Critical Care Medicine, University of Michigan Medical Center, 3916 Taubman Center, Box 0360, Ann Arbor, MI 48109-0360
COPYRIGHT 2000 American College of Chest Physicians
COPYRIGHT 2000 Gale Group