Abstract
Cytarabine, used for the treatment of acute myelogenous leukemia, can cause a rare reaction of inflammation of existing seborrheic keratoses. We report a case of cytarabine induced inflammation of seborrheic keratoses mimicking a vesicular eruption in 53-year-old man with acute myelogenous leukemia.
**********
Case Report
Our patient was a 53-year-old man diagnosed with acute myelogenous leukemia. He was admitted to the hospital for induction chemotherapy with cytarabine and idarubicin. On the fifth day of cytarabine therapy, the patient began to develop fevers and a rash that appeared vesicular (Figure 1) without a clear source of infection.
Acyclovir was initiated by the hospital team for possible disseminated zoster. The fevers persisted despite broad spectrum antibiotics. Cultures were all negative. The dermatology service was consulted to evaluate the patient's rash in relation to his persistent fevers.
On exam, the patient had generalized petechiae, which was a chronic finding secondary to thrombocytopenia. On his upper chest and neck, the patient had multiple 2-4mm tan/white vesicular-like papules on a red base. In addition, on his middle and lower posterior trunk were four similar appearing lesions larger in size (Figure 2).
Attempts to unroof one of the smaller, vesicular-like lesions were unsuccessful. Biopsy demonstrated an inflamed seborrheic keratosis (Figure 3).
[FIGURE 1 OMITTED]
[FIGURE 2 OMITTED]
[FIGURE 3 OMITTED]
Discussion
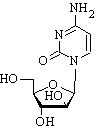
Cytarabine, a nucleoside analogue of deoxycytosine which inhibits DNA synthesis, is primarily used for the treatment of acute myelogenous leukemias and lymphocytic leukemias. More frequent cutaneous side effects include a maculopapular rash and mucositis (1).
The sign of Leser-Trelat is defined as the sudden appearance or enlargement of multiple seborrheic keratoses in association with an internal malignancy (2). Case reports of this sign abound, but the true existence has been called into question, as both seborrheic keratoses and cancer are more common occurrences in the elderly population (3). Two reports have documented an unusual occurrence of cytarabine induced inflammation of existing seborrheic keratoses (4,3), a pseudo-"sign of Leser-Trelat". When this inflammation occurs in smaller seborrheic keratoses, which are common on the anterior chest and around the neck, a vesicular eruption is mimicked. The patient reported by Williams et al. was originally diagnosed with disseminated herpes zoster. In differentiating the inflammation of these smaller seborrheic keratoses from a vesicular eruption, it is helpful to attempt to locate more classical lesions, which were present in our patient.
References
1. Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues and nucleobases in cancer treatment. The Lancet Oncology 2002; 3(7):415-24.
2. Schwartz RA. Sign of Leser-Trelat. J Amer Acad Dermatology 1996. 35(1):88-95.
3. Grob JJ, et al. The relation between seborrheic keratoses and malignant solid tumours. A case-control study. Acta Dermato-Venereologica 1991; 71(2):166-9.
4. Kechijian P, et al. Cytarabine-induced inflammation in the seborrheic keratoses of Leser-Trelat. Ann Intern Med 1979; 91:868-9.
5. Williams JV, Helm KF, Long D. Chemotherapy-induced inflammation in seborrheic keratoses mimicking disseminated herpes zoster. Jour Amer Acad Derm 1999; 40(4):643-4.
TIMOTHY PATTON DO (1), MATTHEW ZIRWAS MD (1), NANCY NIELAND-FISHER MD (2), DRAZEN JUKIC MD PHD (3)
1. DEPARTMENT OF DERMATOLOGY
UNIVERSITY OF PITTSBURGH, PITTSBURGH, PENNSYLVANIA
2. PROFESSOR OF DERMATOLOGY
DEPARTMENT OF DERMATOLOGY
UNIVERSITY OF PITTSBURGH, PITTSBURGH, PENNSYLVANIA
3. DIRECTOR OF DERMATOPATHOLOGY
DEPARTMENT OF DERMATOLOGY
UNIVERSITY OF PITTSBURGH, PITTSBURGH, PENNSYLVANIA
ADDRESS FOR CORRESPONDENCE:
Timothy Patton DO
3601 Fifth Avenue
Fifth Floor
Pittsburgh, PA 15213
Phone: (412) 383-8033
Fax: (412) 648-1962
E-mail: pattontj@msx.upmc.edu
COPYRIGHT 2004 Journal of Drugs in Dermatology, Inc.
COPYRIGHT 2005 Gale Group