ABSTRACT. In the present study, the authors examined the effects of diazepam (Valium) on various physical, social, and emotional variables within the framework of a task designed to test risk-taking behavior. Participants received either 10 mg of diazepam or a placebo. After the participants tasted strong-flavored liquids, they were able to engage in a risky behavior (i.e., drinking from a confederate's "used" water bottle). Half the participants received additional verbal pressure to drink from the bottle. The authors expected that diazepam would increase health-risk behavior, but the results were inconsistent with that prediction. The second goal of the experiment was to explore diazepam's effects on arousal, mood, social anxiety, and taste. Diazepam users exhibited differences in taste perception and social anxiety, which might in part explain the health-risk results.
Key words: benzodiazepines, diazepam, disinhibition, risk taking, social pressure
**********
DRUGS THAT ACT on the neurotransmitter gamma-amino butyric acid (GABA), such as alcohol and diazepam (Valium), are generally classified as anxiolytics because of their ability to reduce anxiety-related behavioral inhibition. Benzodiazepines have been found to exhibit a clear anxiolytic effect upon animals (Kleven & Koek, 1999; Rex, Stephens, & Fink, 1996) and humans (Erdmann, Janke, Neugebauer, & Wolwer, 1993). Among various anxiolytics, benzodiazepines have been especially likely to increase the propensity to disinhibit behaviors (Bond, 1998; Breggin, 1998; Griebel, Belzung, Perrault, & Sanger, 2000), presumably by impairing impulse control and cognitive executive functioning. The results of numerous studies report increases in impulsive responding in animals, such as the disinhibition of open arm exploration after the administration of benzodiazepine (Dalvi & Rodgers, 1999; Laviola, 1996). Benzodiazepines increase impulsive responding in humans (Bond, 1998; Wingrove & Bond, 1997) and release punished responding in conditioned suppression paradigms in which rodents and pigeons are used (Kleven & Koek; McMillan, Li, & Hardwick, 1997; Shumsky & Lucki, 1996).
Drug-induced disinhibition is made possible because of neuronal activity at gamma-amino butyric acid-benzodiazepine (GABA-BZD) receptors (see Breggin, 1998; Griebel et al., 1999). The GABA-BZD receptor complex is a neural substrate that is an important element in an organism's response to environmental stimuli. GABAergic neurotransmission, which is facilitated by benzodiazepines, produces inhibitory central nervous system (CNS) changes. The CNS depression may specifically alter behavior that is controlled by areas in the brain that are replete with benzodiazepine and GABA receptors. Therefore, GABA might facilitate behavioral disinhibition after stimulation at those GABA receptor sites.
Diazepam is a benzodiazepine that is primarily prescribed in clinical populations to treat phobias, anxiety, insomnia, depression, and panic disorders. Researchers have proposed that benzodiazepines help patients with such disorders by reducing their level of behavioral inhibition (Bond, 1998). As benzodiazepines relieve anxiety, there is an overall reduction in socially relevant anticipatory fear associated with the behavioral inhibition system. While benzodiazepines increase social behavior (Corbett et al., 1993; File, 1986; Short & Maier, 1993), they might also result in socially inappropriate behavior. Nitrazepam and flurazepam increase nightmares, hypomania, garrulousness, irritability, and restlessness (see Breggin, 1998, for discussion).
Aggression has been elicited after the ingestion of benzodiazepine in numerous animal trials (File, 1986; Schroeder, Toniolo, Nehlig, & Desor, 1998; Weerts & Miczek, 1996). Likewise, researchers have found that a number of benzodiazepines increase aggressive responding in humans. In particular, consumption of benzodiazepines dose dependently increases the intensity of shock or noise blasts given in the Taylor Reaction-Time Paradigm (RTP; Cherek, Steinberg, Kelly, Robinson, & Spiga, 1990; Gantner & Taylor, 1988; Taylor & Hulsizer, 1998; Weisman, Berman, & Taylor, 1998). The results of studies that use the Taylor RTP show that although benzodiazepines consistently increase aggression in the laboratory, those effects depend on which type of anxiolytic drug is used (Bond & Lader, 1988). Because benzodiazepines seem to influence impulsive and aggressive behavior in the laboratory, those drugs might also disinhibit other behaviors with potentially negative consequences. More specifically, benzodiazepines might precipitate risky health-related behaviors.
Risky behaviors, such as smoking, drinking and driving, unprotected sexual activity and promiscuity, violence, and antisocial behavior have potentially negative health consequences. Indeed, individuals are generally aware of the health threats that exist following such behaviors. Known consequences of risky behaviors (e.g., risk of HIV infection through sexual behavior, risk of illness or injury through drug, alcohol, and tobacco use) are routinely made available to the public in the news media. Yet, health-risk behavior continues to be a major public health threat (Moeller, 1997; Nelson, Sealy, & Schneider, 1993). Furthermore, individual perceptions of health risks and one's susceptibility to a health threat are modified by social and psychological variables (Blanton & Gerrard, 1997).
There is a large body of evidence about environmental, personality, and contextual factors that contribute to the propensity to engage in health-risk behaviors (Blanton & Gerrard, 1997; Connor & Sparks, 1996). The choice to engage in risky health behaviors may be influenced by the consumption of psychoactive substances such as alcohol, cocaine, and heroin. Deas-Nesmith, Brady, White, and Campbell (1999) reported that substance abuse treatment residents, compared with psychiatric patients and healthy controls, were significantly more likely to have engaged in risky sexual behavior, such as oral, vaginal, and anal intercourse without a condom, and sex with a stranger or prostitute, irrespective of knowledge about risky behaviors and the risk of HIV infection. Stiffman, Dore, Cunningham, and Earls (1995) reported that HIV risk behaviors (i.e., unprotected sexual behavior, sharing needles) were in part predicted by substance abuse.
Other supporting evidence for drug-related changes in health-risk behaviors includes a number of clinical and correlational studies (Darke, Ross, & Cohen, 1994; Klee, Faugier, Hayes, Boulton, & Morris, 1990; Lekka, Paschalis, & Beratis, 1997; Ross, Darke, & Hall, 1997). The results of such research indicate that the use of benzodiazepines is associated with an increased likelihood of engaging in polydrug use, including nicotine, caffeine, and alcohol. Lekka et al. found that cigarette smoking, alcohol dependence, and the amount of caffeine used per day was significantly greater among high-dose regular benzodiazepine users. When Lekka and colleagues compared high-dose regular benzodiazepine users with low-dose regular and occasional users and nonusers, they reported a dose-dependent correlation between benzodiazepine use and nicotine and caffeine use. Therefore, the use of benzodiazepines may be a decisive factor contributing to polydrug use.
Recent evidence suggests that individuals who consume benzodiazepines have greater risk for physical and psychosocial illness along with a greater incidence of risky behaviors. Intravenous drug users are more likely to use benzodiazepines and more likely to have increased health problems and social problems than are noninjecting drug users, according to the findings in a study conducted by Darke et al. (1994). The authors suggested that the use of benzodiazepines contributes to the decision to inject a drug. In fact, intravenous drug users reported frequently injecting benzodiazepines and were 3.8 times more likely than were nonbenzodiazepine users to have shared a needle within 1 month before data collection (Ross et al., 1997). The results indicated that benzodiazepine users were at greater risk for disease and infection as a result of poor health behaviors.
The true pharmacological link to risky behaviors cannot be unequivocally assessed because most of the research on risky behaviors and benzodiazepines is correlational. Much of the research on benzodiazepines and health-risk behavior is conducted on clinical populations (i.e., individuals with anxiety disorders and drug addicts). Therefore, research that links drugs to health-risk behaviors might reflect dispositional differences that are unrelated to the consumption of anxiolytic substances. Obviously, investigators need a research paradigm that assesses the direct pharmacological effect of benzodiazepines on health-risk behavior. However, researchers who seek to determine if the consumption of anxiolytics influences risky behaviors cannot ethically design an experiment that allows participants to share needles or engage in risky sexual behavior. A health-risk research paradigm should be able to determine whether risky behaviors are influenced by a benzodiazepine, personality factors, or social constraints. Therefore, in the present research, we used mild, quantifiable health-risk behavior in a controlled setting.
Martin and Leary (1999) developed a laboratory paradigm that measured the propensity to engage in health-risk behaviors: Participants tasted three strongly flavored beverages, after which a confederate offered them a drink from a water bottle from which someone else had already drunk. Sharing beverage containers and silverware increases the likelihood of the transmission of pathogens (Moeller, 1997; Nelson et al., 1993). Because the confederate was a stranger and the participants were not aware of the individual's health status, the propensity to drink out of a stranger's water bottle was considered a risky behavior. Martin and Leary asserted that "other, more serious health-risk behaviors are conceptually similar to the act of sharing a drink. For example, sharing beverage containers and sharing needles for intravenous drug use increases the risk of transmitting diseases from one person to another" (p. 1093).
Martin and Leary (1999) used a manipulation that randomly assigned participants to receive feedback indicating that they had either high or low image concern. They used a verbal challenge condition with half the participants, in which the confederate offered the water bottle and added, "... if you don't mind drinking out of the same bottle as me." The other half of the participants received the standard offer without the added challenge. Martin and Leary found that both assignment to image concern condition and verbal challenge significantly increases the amount of water drunk from the confederate's bottle. The results suggested that both personality variables and interpersonal climate were important in determining what health-related behaviors people would perform. Martin and Leary's research project successfully demonstrated the importance of self-presentation and social influence on health-related outcomes.
The purpose of the present study was to examine the effects of benzodiazepines on health-risk behavior by using a replication of the experimental conditions in the study by Martin and Leary (1999). The design involved the use of a benzodiazepine, diazepam (Valium), to determine whether there was a pharmacological effect on risk taking. We also used the verbal challenge condition from Martin and Leary's experiment to examine the influence of social pressure on health-risk behaviors. According to current literature, the drug should have increased the propensity to drink out of the stranger's bottle. We expected that the verbal challenge would increase the likelihood that the participants would take a risk in a manner that was comparable to that round by Martin and Leary and that considerably more participants would engage in the risky behavior after they had consumed diazepam. In short, the primary hypothesis was that both diazepam and social pressure were sufficient to influence the expression of risky behavior in a social setting.
The design of the present study lent itself to a second empirical examination. Researchers have found that the influence of benzodiazepines increases the palatability of foods (Berridge & Pecina, 1995) and the appetitive and consummatory behaviors in humans and animals (Foltin, 2001 ; Haney, Comer, Fischman, & Foltin, 1997), and reduces conditioned taste aversion in animals (Parker, 1995). There has been little systematic research on the perception of robust gustatory stimulation after diazepam consumption in humans. Thus, in the present study, we aimed to explore how the perception of flavors changed owing to a moderate dose of diazepam.
Method
Participants
The participants were 24 men between the ages of 18 and 25 years. We recruited through sign-up folders that advertised a paid experiment in the department of psychology. No specific details about the study were conveyed at the sign-up table or in the folders. We contacted the volunteers by phone and informed them that the experiment involved the use of a minor tranquilizer and a taste test. They were informed that the procedure would last approximately 2 hr and that they would receive $7 per hr for their participation.
We conducted a preliminary health screening telephonically before we scheduled each participant for participation in the experiment. The purpose of the health screening was to assess past or present illnesses, the use of medications, or the presence of substance use. Potential participants had to be over 18 years old and in good health. Applicants whose responses during the preliminary interview indicated a history of physical or mental illness, present or past liver or kidney disease, food allergies, or lactose intolerance were not scheduled to participate. They were also excluded if they (a) were taking prescription or nonprescription drugs that were contraindicated with diazepam or known to interfere with the metabolism of diazepam, and (b) had a history of excessive substance use or substance abuse treatment. However, the applicants were required to have had at least some experience with alcohol or psychoactive substance use to be considered eligible for participation.
If the general health status questionnaire proved satisfactory, the volunteers were scheduled to participate. We told the volunteers to meet the experimenter (the first author) at the University Health Center. Each participant had to refrain from (a) eating or drinking caffeinated or nutritive beverages for 4 hr before the experiment, (b) smoking for 1 hr before the experiment, and (c) using alcohol or illicit drugs after 9:00 p.m. the night before the experiment.
The participants were treated in accordance with Kent State University Human Subjects Review Board rules and regulations. They were informed that they might receive a dose of a minor tranquilizer. Each participant signed a consent form that explained the possible effects and side effects associated with the use of a minor tranquilizer. Because the experiment involved a taste task, the participants were required to sign a consent form that unambiguously stated that they should not participate if they believed they might have food allergies. The participants were informed that (a) a physician might review their medical history if necessary, (b) the experiment was anonymous, and (c) they could withdraw at any time without penalty.
Materials
Questionnaires. The participants were administered a number of questionnaires throughout the experiment. A detailed health questionnaire was administered to ensure that each participant was healthy and had not indulged in any recent drug or alcohol use. The health questionnaire was also reviewed to avoid confounding due to tolerance. Three participants who had physical or mental-health problems or who had used substances too recently or too frequently were paid for their time and released. The participants were also administered several psychological questionnaires, including the Behavioral Inhibition System-Behavioral Activation System (BIS-BAS) scale (Carver & White, 1994), the Social Desirability Questionnaire (Crowne & Marlowe, 1960), the Stanford Sleepiness Scale (Hoddes, Zarcone, Smythe, Phillips, & Dement, 1973), and a general (unpublished) mood questionnaire. During the experiment, each participant completed an (unpublished) taste-task questionnaire. Finally, following the experiment, all participants completed a general (unpublished) posttask questionnaire as described in BenPorath and Taylor (2002).
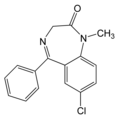
Substances. The drug used in the present study consisted of a 10-mg capsule of diazepam (Valium). The placebo was a capsule of the same size and color as the drug, with a lactose-based nondrug filler.
Samples. The taste task involved the use of three liquid samples that were identical to those used by Martin and Leary (1999). Each contained 15 ml of fluid. The first sample consisted of one part mustard to four parts lemon juice; the second sample consisted of a highly concentrated, sugar-free cherry or lemon KoolAid[R] beverage; and the third sample consisted of plain soy sauce.
Stimulus. The primary stimulus in the health-risk behavior analog task was a single-serving (500-ml) prepackaged bottle of water that would be offered to each participant after tasting the three samples. The water bottle was half full (250 ml) and the label was partially missing to give the appearance that it had been previously consumed. In fact, the bottle was unused.
Procedure
The experimenter greeted the participants in the lobby of the University Health Center and walked to the laboratory facilities. The experimenter asked a series of preliminary questions to ensure that each participant had not eaten, drunk, smoked, or used alcohol or other substances recently. The participants took a breathalyzer test (to be certain they had not consumed alcohol recently), were weighed, and then were escorted to an enclosed cubicle to review the consent forms. While each participant reviewed the consent forms, the experimenter left the room to fetch the other participant (actually a confederate) for the study.
After the participant had signed the consent form, the experimenter administered a detailed health questionnaire to each participant to ensure that the participant was healthy and had not indulged in any recent drug or alcohol use. The latter questionnaire was also reviewed to avoid confounding due to tolerance. Three participants who had physical or mental health problems, or who had used substances too recently or too frequently were paid for their time and released. After the participants had completed the health questionnaire, they filled out several questionnaires, including the Behavioral Inhibition System-Behavioral Activation System (BIS-BAS) scale, the Social Desirability Questionnaire, the Stanford Sleepiness Scale, and the general mood questionnaire. After the first set of questionnaires was completed the drug or placebo was dispensed to the participant in a double-blind fashion. Each participant was randomly selected to receive either a drug or a placebo.
Each participant waited 1 hr for the drug to be absorbed. They were told to read or do homework during that time, and they were not allowed to sleep or listen to music. Those who had no homework to do were given magazines that were first reviewed by the experimenter to ensure they contained only benign information (i.e., containing no articles or ads on health-risk behaviors, bottled water, or drugs). The experimenter frequently observed each participant and documented any noticeable changes in mood, fatigue, or activity level of the participant. After 1 hr, a rigged drawing was conducted to ensure that the participant would be "Participant A" (the taste tester) and the confederate would be "Participant B" (the rater).
The participant completed the second mood and sleepiness questionnaire and entered the experimental room. The confederate then entered the experimental room, carrying a backpack that contained the primary stimulus item used to test health-risk behavior. Half the confederates used were men and the other half were women. The backpack contained the single-serving, prepackaged, half-full water bottle.
The taste task involved the use of three liquid samples, identical to those used by Martin and Leary (1999). The experimenter instructed the participant how to taste each of the three beverages and rate the tastes. The "taster" (participant) was told to place the beverage in his mouth and hold it there for 5 s and then to swallow it, after which he had to answer all the questions on the taste rating sheet. The rating sheet contained 9 items designed to assess a number of dimensions of taste (i.e., sweetness, saltiness, enjoyableness) and a 10th item in which the participant had to guess what was in the sample. The order of the beverages was randomized and counterbalanced. The "rater" (confederate) was told to observe the facial expressions made by the other participant and to write down anything he noticed on a rating sheet. Each confederate was told to record what the sample may have tasted like. The experimenter left the room and watched the task from a separate room through a two-way mirror. That served two purposes. First, the experimenter paid close attention to the timing and details of the taste task to ensure that the confederate followed procedure and used the exact script. Second, the experimenter was readily available should any problems have arisen (e.g., choking, allergic reactions) during the taste task. However, no taste-task sessions were terminated because of any such problems.
After the participant finished the last beverage and the last taste questionnaire, the confederate pulled out the half-full bottle of water and said, "That stuff must've tasted pretty nasty. Do you want a drink of my water?" In the challenge condition, the confederate added "... that is, if you don't mind drinking out of the same bottle as me." Each script was identical to that used by Martin and Leary (1999). The two participants then finished the questionnaires, which consisted of a posttask questionnaire and a final mood and sleepiness questionnaire, in the experimental room. After all the questionnaires had been completed, the participant was led out of the experimental room, paid, debriefed, and escorted to the health center exit. The experimenter then measured how much water, if any, the participant had drunk.
Results
The main hypothesis in the present experiment was that benzodiazepines would increase the propensity to engage in a risky behavior. The results were not consistent with that hypothesis. A second goal of the present experiment was to explore effects on taste, mood, and social anxiety after the ingestion of diazepam. It is interesting to note that the diazepam users reported differences in those three measures, which might in part explain the inconsistent results pertaining to health-risk behavior.
Manipulation Check
A chi-square analysis revealed that the participants' internal drug states were accurately detected by the majority of participants, [chi square](1, N = 24) = 15.683, p < .001. The participants in the drug condition reported feeling that they received higher amounts of the drug than those in the placebo condition, F(1, 23) = 4.179, p < .05, and reported feeling greater effects of the drug, F(1, 23) = 12.397, p < .002.
Health-Risk Behavior
A chi-square analysis revealed a significant overall propensity to drink from the bottle regardless of condition. Most participants drank from the confederate's bottle, [chi square](1, N = 24) = 6.279, p [less than or equal to] .01. We performed a log-linear analysis to determine if there were differences in the number of participants who drank from the confederate's bottle (see Table 1) as a function of drug and challenge. The log-linear analysis revealed a main effect of drug on propensity to drink from the bottle, [chi square](1, N = 24) = 3.807, p [less than or equal to] .05. Challenge did not have a significant effect on propensity to drink, [chi square](1, N = 24) = .902, p > .05. However, the interaction between drug and challenge reached significance, [chi square](1, N = 24) = 3.684, p [less than or equal to] .05, such that more participants drank from the bottle after a verbal challenge if they received a placebo than if they received a drug. We conducted a power analysis to determine if sample size was sufficient to detect drug effects on the frequency data. We used a normal approximation for a log method of computation and found that power for drug effects was underutilized (48%). Our significant findings suggested that by Cohen (1988) conventions, the effect size was robust.
We performed a 2 x 2 ANOVA to assess the effect of drug and challenge on the mean amount of water drunk from the confederate's bottle. We found no main effects for diazepam, F(1, 20) = .939, p > .05, or challenge, F(1, 20) = .839, p > .05, on amount of water drunk. We found no interaction between drug and challenge, F(1, 20) = 2.258, p > .05, on amount of water drunk.
Self-Presentation, Mood, and Arousal
We calculated self-presentational concern from the Social Desirability questionnaire (Crowne & Marlowe, 1960). The participants' scores on that questionnaire were not correlated with amount of water drunk from the water bottle, r = .191. When the participants were assigned to one of three categories on the basis of their social desirability scores (high, medium, low), slight differences emerged in propensity to drink from the bottle. Both high and medium scorers drank from the confederate's bottle in 7 out of 8 cases. In the low scoring group, only 4 out of 8 participants drank from the bottle. Those proportions did not reach significance. Social desirability combined with drug state or challenge did not predict propensity to drink (all ps > .05).
Another measure of self-presentation was taken from the posttask assessment, which revealed an effect of diazepam on participants' concern over what the experimenter thought of them, F(1, 22) = 6.11, p [less than or equal to] .02, as well as concern over what the other participant (confederate) thought of them, F(1, 21) = 7.025, p [less than or equal to] .02. It is interesting to note that those who received a drug were more likely to endorse concern over the experimenter's opinion and the other participant's opinion than were those who received a placebo.
The experimenters used the general mood questionnaire and the Stanford Sleepiness Scale to assess anxiety, anger, arousal, and sleepiness before drug consumption, 1 hr after drug administration, and immediately after the taste test. We conducted a 2 x 2 x 3 repeated-measures ANOVA on each measure as a function of drug, challenge, and time of assessment. There were no significant differences between drug and placebo conditions on anxiety, anger, or arousal, all ps > .05. It was not surprising that a significant effect was found for diazepam on measures of sleepiness, F(1, 18) = 17.121, p < .001, such that participants reported higher levels of sleepiness after taking the drug compared with after taking the placebo.
Mood and arousal measures changed as a function of time of assessment. Anxiety, F(2, 34) = 4.975, p < .01, anger, F(2, 38) = 5.367, p < .01, and sleepiness, F(2, 36) = 13.178, p < .001, but not arousal (p > .05), changed as a function of assessment, independent of drug condition. Anxiety measured at time 2 significantly dropped from baseline, t(20) = 3.170, p < .01 (two-tailed), d = 1.16, and then increased significantly from time 2 to time 3, t(22) = -2.665, p < .01 (two-tailed), d = 1.35. Anger rose significantly from baseline to time 3, t(23) = -2.627, p < .01 (two-tailed), d = 1.00, and from time 2 to time 3, t(22) = 2.44, p < .05 (two-tailed), d = 0.69. That reflected increased anxiety and anger after the taste-task portion of the experiment. All the participants experienced an increase in sleepiness 1 hr after taking either the drug or the placebo, F(2, 36) = 13.178, p < .001, followed by a significant decrease in sleepiness from time 2 to time 3, t(21)= 3.521, p < .01 (two-tailed), d = 0.55, showing increased arousal surrounding the taste task.
Taste
After the participants tasted each sample, they completed a 9-item taste ratings sheet that rated the tastes on a variety of dimensions, each using a 10-point scale. The taste qualifies sweetness, saltiness, sourness, and bitterness were measured, as well as measures of palatability and disgust for the beverages. We conducted A 2 x 3 ANOVA to test for differences in taste ratings as a function of drug and each sample. An examination of differences between the three samples revealed significant effects of sample type overall, such that each beverage received quantitatively different scores across questionnaire items (all ps < .01). For example, the Kool-Aid[R] beverage received the highest ratings on sweetness, the mustard-lemon beverage received the highest ratings on the dimensions of bitter, and soy sauce had the highest ratings on robustness and disgust. Comments on the taste-task questionnaire revealed that most participants found the samples unpleasant, particularly the soy sauce. When the experimenter asked a participant to guess the contents of the soy sample, the participant guessed it might contain "runoff from garbage." In fact, although facial expressions were not recorded when the experimenter viewed the taste-test session, the experimenter noted, anecdotally, that participants often gagged, squinted, and covered their mouths with tissues while they drank the beverages.
Ratings for saltiness, sourness, and bitterness did not differ as a function of the drug (all ps > .05). Yet, ratings of sample palatability appeared to have changed as a function of drug state (see Figure 1). Diazepam had a significant effect on how sweet the samples were perceived as being, F(1, 22) = 5.241, p [less than or equal to] .03. Those under the influence of the drug rated the samples as sweeter than did those who had a placebo. Also, the disgust dimension differed between the drug and placebo conditions, such that the participants who consumed the drug had lower subjective disgust measures than did those who consumed a placebo. That effect approached significance, F(1, 22) = 3.893, p [less than or equal to] .06.
[FIGURE 1 OMITTED]
Discussion
The primary goal of the present study was to determine if diazepam would influence health-risk behaviors. The main expectation was that diazepam would increase risk-taking behavior, as predicted from the current literature on benzodiazepines and behavioral disinhibition. Researchers have shown that benzodiazepines disinhibit other potentially hazardous behaviors within the context of social interactions (Bond & Lader, 1988; Griebel et al., 2000; Lekka et al., 1997; Schroeder et al., 1998; Weerts & Miczek, 1996). Yet, the results of the present study revealed that diazepam reduced the amount of risk-taking behavior, which was demonstrated by the finding that those in the placebo condition were more likely to drink from the confederate's bottle than were those in the drug condition. However, that result was almost entirely owing to effects found in the drug-challenge condition. Participants were more likely to drink from the stranger's bottle if they were under the influence of the drug, but only if they received the verbal challenge. The results of the present study did not support our original expectations.
One possible conclusion for the findings is that benzodiazepines do not disinhibit health-risk behaviors. However, there is considerable evidence in the literature that benzodiazepines reliably disinhibit risky behaviors. The results of the present study seem to be incongruous with correlational studies on benzodiazepines and health risks (Griebel et al., 2000). We expect diazepam to have anxiolytic properties at peak blood plasma levels (approximately 1 hr after ingestion). Perhaps we did not succeed in the present experiment in producing anxiolytic drug effects that were clearly discriminable from nondrug states. Diazepam did not produce consistent effects on anxiety. The anxiety measurement, taken at three time points, did not change as a function of drug state, yet during the post-task questionnaire, the participants who were under the influence of the drug reported more concern over what both the experimenter and the other participant thought of them. Those findings showed that the drug elevated social anxiety but not generalized feelings of anxiety. Instead, anxiety changed as a function of time of assessment, regardless of assignment to drug condition. Once the participants were in the taste-test portion of the experiment, all of them reported increased levels of anxiety. The taste-test procedure was sufficiently stressful to increase the ratings of anxiety across the drug conditions, whereas the diazepam dose was not sufficient enough to counter the participants' feelings of anxiety.
Most of the participants (18 out of 24) chose to drink from the confederate's bottle. One possible explanation for that finding is that the experimental design made it less likely for the participants to refuse the drink. The confederate who was used in the analog task is considered a "stranger." However, the design of the present experiment may have inadvertently created a false sense of familiarity with the confederate in the participant. Although the confederate and participant were kept in separate cubicles, the participants were aware that there was another person who was going through the same procedure, including the health screening, the breathalyser test, and drug or placebo consumption. They may have thought that the other person was experiencing similar internal feelings (e.g., apprehension, sedation, boredom) during the experiment. Both the participant and confederate were positioned in the same general vicinity for more than 1 hr before the taste test, whereas the participants in the experiment by Martin and Leary (1999) were in the same vicinity for only a few moments. Therefore, the participants in the present experiment might have felt more familiar with their taste-task partner than did Martin and Leary's participants.
The interpretation of the results of the present experiment should not disregard benzodiazepine's effect on taste perception. In the present study, we found differences in taste perception as a function of drug state, and those findings are consistent with other research on various benzodiazepines (Berridge & Pecina, 1995). Parker (1995) found that chlordiazepoxide reduced conditioned taste aversion and enhanced hedonic ingestive reactions in rats. Foltin (2001) found that diazepam dose dependently increased food-intake (both appetitive and consummatory) behaviors in baboons. Haney et al. (1997) reported that alprazolam increased food consumption and daily caloric intake in humans owing to significant increases in the number of eating sessions per day and decreases in intersession intervals.
Given such findings, the increase in sweetness and reduced disgust reported by the participants in the present experiment was not surprising. The participants who had the drug reported less disgust in response to all of the samples and reported higher ratings of sweetness for samples. Therefore, the reduced propensity to drink might simply have reflected the fact that individuals who consumed the drug had less cause to ameliorate the intense displeasure caused by the samples and were, therefore, less likely to drink from the bottle. However, rates of drinking in the drug-no challenge group are nearly identical to each of the placebo groups. Given that observation, sample palatability is unlikely to be the sole cause for the participants' refusal.
Still, the ability of diazepam to influence social behavior could explain the results of the present study. Only those participants who received the drug and the verbal challenge were more likely to refuse the drink offer. Perhaps when the confederate offered the drink to the participant, the use of the scripted phrase "... that is, if you don't mind drinking out of the same bottle as me," actually drew attention to the risky nature of the drinking behavior. The challenge could have served as peer pressure for participants in the placebo condition, whereas the same manipulation was a reminder of risk for participants in the drug condition. Sharing a bottle of much-appreciated water with a complete stranger might seem benign without the verbal challenge, but it suddenly appears risky when the challenge itself suggests that sharing a bottle is in some way unsavory. It would be interesting to see if similar drug effects would have emerged if the peer-pressure manipulation had been refined to either enhance and/or eliminate that type of contextual cue.
One needs to consider sample palatability, anxiolytic effects of the drug, and the effects of social pressure when explaining the present study's findings. The inconsistent findings may indeed be consistent with experiments testing independent effects of benzodiazepines. Therefore, the refusal of the participants in the drug-challenge condition might be a result of the combined effect of (a) increased palatability and less disgust for the flavors, (b) the cue established by the verbal challenge, and (c) the reduction in anxiety that allowed the participants to risk the social consequences of their rejection of the bottle.
An obvious limitation of the present study was the low number of participants in each condition. Because of the insufficient power, it would be premature to draw firm conclusions from null results, given the increased risk for a Type II error. Furthermore, the results cannot generalize to a broad population of people because we studied only young, healthy men. A different effect might have emerged had our study included women. Studies show that men and women are likely to take different risks under the influence of drugs (Byrnes, Miller, & Schafer, 1999; Schmid, 1998). Women are more likely to drive under the influence of alcohol, whereas men are more likely to engage in risky sexual behaviors after alcohol consumption than are women. Therefore, we need to replicate the taste-task paradigm to determine if the paradigm will be a reliable way of measuring risk-taking behaviors across different situations with different populations. Also, we need a follow-up study to establish how benzodiazepine use and social pressure combine to influence risky health-related behaviors.
Ways in which we could improve the design of the experiment include (a) changing the context to reflect a more naturalistic setting, (b) reducing the level of familiarity between the participant and the confederate, and (c) making the health risks more salient. Perhaps benzodiazepines would have disinhibited risky behavior within the laboratory if a different behavioral measure had been used, such as accepting an offer to try a new "drug," meeting a stranger for a possible date, or engaging in sensation-seeking behaviors (e.g., skydiving). Such realistic risky behaviors would be difficult to elicit in a controlled laboratory setting. The analog task designed by Martin and Leary (1999) might need to be refined so that the direct effects of anxiety, social constraints, or self-presentation on risky behavior can be unequivocally determined.
Conclusions
Benzodiazepines have long been prescribed as anxiolytics, and they are presently the drug of choice for clinicians who treat anxiety disorders. In the current literature, researchers report that benzodiazepines seem to influence the disinhibition of social behaviors, including aggression, sexual risk taking, and poly-drug use. Continued research is needed to determine if benzodiazepines can reduce social anxiety and whether the anxiolytic action of the drug causes the disinhibition of risky behaviors during social interactions. In the present experiment, we found that disinhibition in a social context could take the form of behaviors that actually resist social pressures to engage in unwanted activities when under the influence of an anxiolytic drug. Although the results did not demonstrate any appreciable risk behaviors as a function of diazepam use, the results are potentially useful for comparing effects of benzodiazepines on other socially relevant behaviors. More controlled research is needed to determine the role of substance use, anxiety, and individual differences on social behaviors and health outcomes.
REFERENCES
Ben-Porath, D. D., & Taylor, S. P. (2002). The effects of diazepam (Valium) and aggressive disposition on human aggression: An experimental investigation. Addictive Behaviors, 27, 167-177.
Berridge, K. C., & Pecina, S. (1995). Benzodiazepines, appetite, and taste palatability. Neuroscience and Biobehavioral Reviews, 19, 121-131.
Blanton, H., & Gerrard, M. (1997). Effect of sexual motivation on men's risk perception for sexually transmitted disease: There must be 50 ways to justify a lover. Health Psychology, 16, 374-379.
Bond, A. J. (1998). Drug-induced behavioral disinhibition. CNS Drugs, 9, 41-57.
Bond, A. J., & Lader, M. H. (1988). Differential effects of oxazepam and lorazepam on aggressive responding. Psychopharmacology, 95, 369-373.
Breggin, P. R. (1998). Analysis of adverse behavioral effects of benzodiazepines with a discussion on drawing scientific conclusions from the FDA's spontaneous reporting system. The Journal of Mind and Behavior, 19, 21-50.
Byrnes, J. P., Miller, D. C., & Schafer, W. D. (1999). Gender differences in risk taking: A meta-analysis. Psychological Bulletin, 125, 367-383.
Carver, C. S., & White, T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS-BAS scales. Journal of Personality and Social Psychology, 67, 319-333.
Cherek, D. R., Steinberg, J. L., Kelly, T. H., Robinson, D. E., & Spiga, R. (1990). Effects of acute administration of diazepam and D-amphetamine on aggressive and escape responding of normal male subjects. Psychopharmacology, 100, 173-181.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum.
Corbett, R., Hartman, H., Kerman, L., Woods, A., Strupczewski, J., Helsley, G., et al. (1993). Effects of atypical antipsychotic agents on social behavior in rodents. Pharmacology, Biochemistry and Behavior, 45, 9-17.
Connor, M., & Sparks, P. (1996). The theory of planned behavior and health behaviors. In M. Connor & P. Norman (Eds.). Predicting health behavior: Research and practice with social cognition models (pp. 121-162). Philadelphia: Open University Press.
Crowne, D., & Marlowe, D. (1960). A new scale of social desirability independent of psychopathology. Journal of Consulting Psychology, 24, 349-354.
Dalvi, A., & Rodgers, R. J. (1999). Behavioral effects of diazepam in the murine plusmaze: Flumazenil antagonism of enhanced head dipping but not the disinhibition of open-arm avoidance. Pharmacology, Biochemistry and Behavior, 62, 727-734.
Darke, S., Ross, J., & Cohen, J. (1994). The use of benzodiazepines among regular amphetamine users. Addiction, 89, 1683-1690.
Deas-Nesmith, D., Brady, K. T., White, R., & Campbell, S. (1999). HIV-risk behaviors in adolescent substance abusers. Journal of Substance Abuse Treatment, 16, 169-172.
Erdmann, G., Janke, W., Neugebauer, S., & Wolwer, W. (1993). On anxiety-specific actions of tranquilizers. Anxiety, Stress, and Coping, 6, 25-42.
File, S. E. (1986). Effects of neonatal administration of diazepam and lorazepam on performance of adolescent rats in tests of anxiety, aggression, learning, and convulsions. Neurobehavioral Toxicology and Teratology, 8, 301-306.
Foltin, R. W. (2001). Effects of amphetamine, dexfenfluramine, diazepam, and other pharmacological and dietary manipulations on food "seeking" and taking behavior in nonhuman primates. Psychopharmacology, 158, 28-38.
Gantner, A. B., & Taylor, S. P. (1988). Human physical aggression as a function of diazepam. Personality and Social Psychology Bulletin, 14, 479-484.
Griebel, G., Perrault, G., Letang, V., Granger, F., Avanet, P., Schoemaker, H., et al. (1999). New evidence that the pharmacological effects of benzodiazepine receptor ligands can be associated with activities at different BZ (w) receptor subtypes. Psychopharmacology, 146, 205-213.
Griebel, G., Belzung, C., Perrault, G., & Sanger, D. J. (2000). Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology, 148, 164-170.
Haney, M., Comer, S. D., Fischman, W., & Foltin, R. W. (1997). Alprazolam increases food intake in humans. Psychopharmacology, 132, 311-314.
Hoddes, E., Zarcone, V., Smythe, H., Phillips, R., & Dement, W. C. (1973). Quantification of sleepiness: A new approach. Psychophysiology, 10, 431-436.
Klee, H., Faugier, J., Hayes, C., Boulton, T., & Morris, J. (1990). AIDS-related risk behavior, polydrug use and temazepam. British Journal of Addiction, 85, 1125-1132.
Kleven, M. S., & Koek, W. (1999). Effects of benzodiazepine agonists on punished responding in pigeons and their relationship with clinical doses in humans. Psychopharmacology, 141, 206-212.
Laviola, G. (1996). On mouse pups and their lactating dams: Behavioral consequences of early exposure to oxazepam and interacting factors. Pharmacology, Biochemistry and Behavior, 55, 459-474.
Lekka, N. P., Paschalis, C., & Beratis, S. (1997). Nicotine, caffeine, and alcohol use in high- and low-dose benzodiazepine users. Drug and Alcohol Dependence, 45, 207-212.
Martin, K. A., & Leary, M. R. (1999). Would you drink after a stranger? The influence of self-presentational motives on willingness to take a health risk. Personality & Social Psychology Bulletin, 25, 1092-1100.
McMillan, D. E., Li, M., & Hardwick, W. C. (1997). Discriminative stimulus effects and antipunishment effects of drugs measured during the same session. Pharmacology, Biochemistry and Behavior, 56, 161-166.
Moeller, J. L. (1997). Aseptic meningitis: A seasonal concern. Physician and Sports Medicine, 25, 35-42.
Nelson, S., Sealy, D. P., & Schneider, E. F. (1993). The aseptic meningitis syndrome. American Family Physician, 48, 809-815.
Parker, L. A. (1995). Clordiazepoxide enhances the palatability of lithium-, amphetamine, and saline-paired saccharin solution. Pharmacology, Biochemistry and Behavior, 50, 345-349.
Rex, A., Stephens, D. N., & Fink, H. (1996). "Anxiolytic" action of diazepam and abecarnil in a modified open field test. Pharmacology, Biochemistry and Behavior, 53(4), 1005-1011.
Ross, J., Darke, S., & Hall, W. (1997). Transitions between routes of benzodiazepine administration among heroin users in Sydney. Addiction, 92, 697-705.
Schmid, H. (1998). Female and male students' intention to accept illicit drugs as a function of their risk perception and risk taking. Swiss Journal of Psychology, 57, 47-56.
Schroeder, H., Toniolo, A. M., Nehlig, A., & Desor, D. (1998). Long-term effects of early diazepam exposure on social differentiation in adult male rats subjected to the diving-for-food situation. Behavioral Neuroscience, 112, 1209-1217.
Short, K. R., & Maier, S. F. (1993). Stressor controllability, social interaction, and benzodiazepine systems. Pharmacology, Biochemistry and Behavior, 4, 827-835.
Shumsky, J. S., & Lucki, I. (1996). Differential tolerance to the effects of chlordiazepoxide on unpunished and punished operant responding following chronic treatment. Pharmacology, Biochemistry and Behavior, 53, 593-601.
Stiffman, A. R., Dore, P., Cunningham, R. M., & Earls, F. (1995). Person and environment in HIV risk behavior change between adolescence and young adulthood. Health Education Quarterly, 22, 211-226.
Taylor, S. P., & Hulsizer, M. R. (1998). Psychoactive drugs and human aggression. In R. G. Geen & E. Donnerstein (Eds.), Human aggression: Theories, research, and implications for social policy (pp. 139-165). San Diego, CA: Academic Press.
Weerts, E. M., & Miczek, K. A. (1996). Primate vocalizations during social separation and aggression: Effects of alcohol and benzodiazepines. Psychopharmacology, 127, 255-264.
Weisman, A. M., Berman, M. E., & Taylor, S. P. (1998). Effects of clorazepate, diazepam, and oxazepam on a laboratory measurement of aggression in men. International Clinical Psychopharmacology, 13, 183-188.
Wingrove, J., & Bond, A. J. (1997). Impulsivity--a state as well as trait variable: Does mood awareness explain low correlations between trait and behavioural measures of impulsivity? Personality and Individual Differences, 22, 333-339.
ELIZABETH E. CALDWELL
Department of Psychology
Kent State University
PATRICIA WALLACE
Department of Psychology
University of Lethbridge, Canada
STUART P. TAYLOR
Department of Psychology
Kent State University
Address correspondence to Elizabeth E. Caldwell, Department of Psychology, Kent State University, 118 Kent Hall, Kent. OH 44242; ecaldwel@kent.edu (e-mail).
Manuscript received April 10, 2002
Revision accepted for publication April 2, 2003
COPYRIGHT 2004 Heldref Publications
COPYRIGHT 2004 Gale Group