Glucagon, secreted from pancreatic [alpha]-cells integrated within the islets of Langerhans, is involved in the regulation of glucose metabolism by enhancing the synthesis and mobilization of glucose in the liver. In addition, it has other extrahepatic effects ranging from lipolysis in adipose tissue to the control of satiety in the central nervous system. In this article, we show that the endocrine disruptors bisphenol A (BPA) and diethylstilbestrol (DES), at a concentration of [10.sup.-9] M, suppressed low-glucose--induced intracellular calcium ion ([[[Ca.sup.2+]].sub.i]) oscillations in [alpha]-cells, the signal that triggers glucagon secretion. This action has a rapid onset, and it is reproduced by the impermeable molecule estradiol ([E.sub.2]) conjugated to horseradish peroxidase (E-HRP). Competition studies using E-HRP binding in immunocytochemically identified [alpha]-cells indicate that 17[beta]-[E.sub.2], BPA, and DES share a common membrane-binding site whose pharmacologic profile differs from the dassical ER. The effects triggered by BPA, DES, and [E.sub.2] are blocked by the G[alpha]i- and G[[alpha].sub.o]-protein inhibitor pertussis toxin, by the guanylate cyclase-specific inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, and by the nitric oxide synthase inhibitor N-nitro-L-arginine methyl ester. The effects are reproduced by 8-bromo-guanosine 3',5'-cyclic monophosphate and suppressed in the presence of the cGMP-dependent protein kinase inhibitor KT-5823. The action of [E.sub.2], BPA, and DES in pancreatic [alpha]-cells may explain some of the effects elicited by endocrine disruptors in the metabolism of glucose and lipid. Key words: cGMP, endocrine disruptors, environmental estrogens, estrogen receptors, glucagon, islets of Langerhans, nongenomic, second messengers. doi:10.1289/ehp.8002 available via http://dx.doi.org/[Online 18 May 2005]
**********
Glucagon is a 29-amino acid pancreatic hormone that is secreted from the pancreatic [alpha]-cells into the portal blood supply in response to hypoglycemia, acting as the counter-regulatory hormone to insulin. Its main biologic effect is the regulation of glucose metabolism by enhancing the synthesis and mobilization of glucose in the liver. There is solid evidence demonstrating that the inhibition of glucagon signaling in vivo leads to a reduction of plasma glucose (Jiang and Zhang 2003). In addition, glucagon has many extrahepatic effects, such as the increase of lipolysis in adipose tissue, a positive inotropic effect in the heart, a role in the satiety control in the central nervous system, and the regulation of the glomerular filtration rate (Berne and Levy 1993). In the islets of Langerhans, it participates in the regulation of intraislet hormone, insulin, somatostatin, and glucagon secretion (Gromada et al. 1997a; Ma et al. 2005). When insulin secretion from [beta]-cells is impaired, diabetes mellitus develops. In this pathology, the normal physiologic suppression of glucagon secretion from pancreatic [alpha]-cells in response to elevated plasma glucose is lost (Unger and Orci 1981a, 1981b). A concomitant decrease in early insulin secretion in response to oral glucose is also observed. These combined defects alter the insulin-to-glucagon ratio, leading to a failure of the normal suppression of endogenous glucose production that occurs after ingestion of oral glucose (Gerich 1997). This contributes to the elevation of glucose levels in plasma in individuals with impaired glucose tolerance or diabetes.
Despite the great importance of [alpha]-cells, little is known about the stimulus secretion coupling and its regulation by other hormones and neurotransmitters. This is partly due to the scarcity of islet tissue and small proportion of [beta]-cells compared with insulin-releasing [alpha]-cells. [alpha]-Cells contain a specific set of ion channels, including a voltage-dependent [Na.sup.+] channel, responsible for their electrical activity (Gopel et al. 2000; Rorsman and Hellman 1988; Salehi et al. 1996). Consequently, the intracellular calcium ion [[[Ca.sup.2+]].sub.i] oscillates at low glucose concentrations (Berts et al. 1996; Nadal et al. 1999). Because of the calcium influx, the exocytotic machinery is initiated and glucagon is released (Gopel et al. 2004; Gromada et al. 1997b). When the extracellular glucose concentration increases to the level needed for insulin to be released, the frequency of [[[Ca.sup.2+]].sub.i] oscillations diminishes and, as a consequence, glucagon release decreases (Nadal et al. 1999; Opara et al. 1988).
Although the endocrine pancreas is not considered a classic estrogen target, estrogen effects on insulin and glucagon secretion and receptors of a classical and nonclassical nature are present in islet cells (Nadal et al. 2004; Sutter-Dub 2002). [[[Ca.sup.2+]].sub.i] oscillations have recently been described as finely regulated by 17[beta]-estradiol (17[beta]-[E.sub.2]) in both [alpha]- and [beta]-cells. In the glucagon-containing [alpha]-cells, 17[beta]-[E.sub.2] provokes the suppression of [[[Ca.sup.2+]].sub.i] oscillations generated by low glucose, whereas in [beta]-cells the gonadal hormone potentiates [Ca.sup.2+] signals (Ropero et al. 2002). In both types of cells the 17[beta]-[E.sub.2] effect is initiated after binding to a nonclassical membrane estrogen receptor (ncmER) (Nadal et al. 2000; Ropero et al. 2002).
Environmental estrogens are endocrine-disrupting chemicals (EDCs) that in many cases imitate the actions of the natural hormone 17[beta]-[E.sub.2], eliciting deleterious effects on humans and wildlife (Colborn et al. 1996; Guillette et al. 1996; Hayes et al. 2002; Hunt et al. 2003). It is well accepted that EDCs exert their effects after binding at the classical ER-[alpha] and ER-[beta], inducing classic nuclear actions (Grunfeld and Bonefeld-Jorgensen 2004; McLachlan 2001). Most of the effects described through the classical pathway occur at micromolar concentrations of EDCs. Evidence accumulated in the last few years indicates that EDCs imitate 17[beta]-[E.sub.2] actions through alternative pathways, which in some cases are unrelated to classical ERs (McLachlan 2001; Nadal et al. 2005; Witorsch 2002). Some of these pathways are activated at picomolar and nanomolar concentrations of EDCs (Bulayeva and Watson 2004; Nadal et al. 2000; Quesada et al. 2002; Wozniak et al. 2005).
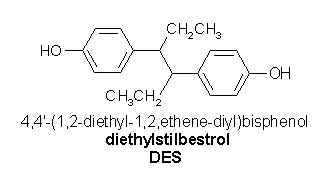
We have described in pancreatic [beta]-cells the effect of two EDCs: bisphenol A (BPA), found in polycarbonate plastic, in the content of food in coated cans, dental sealant, and composites (Brotons et al. 1995; Sonnenschein and Soto 1998), and diethylstilbestrol (DES), a synthetic estrogen used between the 1940s and the 1970s to prevent miscarriages (Newbold 2004). Both bind to an ncmER to induce [[[Ca.sup.2+]].sub.i] signals at concentrations as low as [10.sup.-9] M (Nadal et al. 2000). In addition, [10.sup.-9] M BPA via the ncmER activates the transcription factor CREB (Quesada et al. 2002).
In pancreatic [beta]-cells, we used laser scanning confocal microscopy to study [[[Ca.sup.2+]].sub.i] movements as an index of changes in intracellular signaling because [[[Ca.sup.2+]].sub.i] signals are involved in a large number of signaling processes, from secretion to gene expression (Quesada and Soria 2004; Soria et al. 2004). We used pancreatic [alpha]-cells within freshly isolated islets of Langerhans, the physiologic unit of the endocrine pancreas. This preparation is similar to the actual physiologic situation as demonstrated by [[[Ca.sup.2+]].sub.i] and electrical activity recordings in vivo (Fernandez and Valdeolmillos 2000; Sanchez-Andres et al. 1995). Moreover, this methodology excels when applied to this particular type of cell, which is very difficult to work with because of its scarcity ([alpha]-cells represent only about 10-20% of the total number of islet cells, i.e., ~ 150-300 cells per islet). Using this approach, we demonstrated that low doses of BPA and DES imitate the [E.sub.2]-induced blockade of [Ca.sup.2+] signals in glucagon-releasing [alpha]-cells within intact islets of Langerhans. This effect is mediated by the interaction with an ncmER and involves G-proteins, nitric oxide synthase (NOS), and cGMP-dependent protein kinase (PKG).
Materials and Methods
Materials. We obtained Fluo-3 AM from Molecular Probes Inc. (Leiden, the Netherlands); ICI182,780 and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) from Tocris Cookson Ltd. (Avonmouth, UK); and KT-5823 from Alomone Labs (Jerusalem, Israel). Other chemicals were obtained from Sigma (Madrid, Spain). We also obtained estradiol--horseradish peroxidase (E-HRP) from Sigma; however, it is not available from this company at the present time.
Measuring intracellular calcium in [alpha]-cells within intact islets of Langerhans. Swiss albino OF1 male mice (8-10 weeks of age) were killed by cervical dislocation according to national guidelines provided by our animal house. An internal animal care and use committee reviewed and approved the method used.
Pancreatic islets of Langerhans were isolated by collagenase digestion as previously described (Nadal et al. 1998) and loaded with 5 [micro]M Fluo-3 AM for at least 1 hr at room temperature. Loaded islets were kept in a medium containing 115 mM NaCl, 10 mM NaHC[O.sub.3], 5 mM KCl, 1.1 mM Mg[Cl.sub.2], 1.2 mM Na[H.sub.2]P[O.sub.4], 2.5 mM Ca[Cl.sub.2], and 25 mM HEPES, plus 1% albumin and 5 mM D-glucose, continuously gassed with a mixture of 95% [O.sub.2] and 5% C[O.sub.2] (pH 7.35). Islets were perfused at a rate of 1 mL/min with a modified Ringer solution containing 120 mM NaCl, 5 mM KCl, 25 mM NaHC[O.sub.3], 1.1 mM Mg[Cl.sub.2], and 2.5 mM Ca[Cl.sub.2] (pH 7.35 when gassed with 95% [O.sub.2] and 5% C[O.sub.2]). It took 30-60 sec to change the bath volume completely; this may explain the different response times for some of the experiments.
Calcium records in individual cells were obtained by imaging intracellular calcium under a Zeiss PASCAL5 confocal microscope using a Zeiss 40x oil-immersion lens, with numerical aperture 1.3 (Zeiss, Jena, Germany). Images were collected at 2-sec intervals, and the time course of fluorescent signals from individual cells were measured using the Zeiss LSM software package (Zeiss, Heidelberg, Germany). Experiments were performed at 34[degrees]C. Results were plotted using commercially available software (Sigmaplot; Jandel Scientific, Erkrath, Germany) in which the change in fluorescence ([DELTA]F) is expressed as a percentage of the basal fluorescence ([F.sub.0]) observed in the absence of stimulus or during the intervals between spikes. [alpha]-Cells within the islets were identified by their [[[Ca.sup.2+]].sub.i] oscillatory pattern in 0.5 mM glucose (Nadal et al. 1999; Quesada et al. 1999). The frequency of [[[Ca.sup.2+]].sub.i] oscillations was calculated during a period of 2-4 min before stimuli application (control), and the frequency was measured during 5 min of stimuli application calculated over the period from minute 5 to minute 10 after stimuli were applied. Because a stable baseline before stimuli application was nonexistent, a spike was defined by a rapid increase in [[[Ca.sup.2+]].sub.i] higher than twice the SD of background signals at the intervals between spikes. Spikes were counted manually.
Cell isolation and culture. Isolated islets were dispersed into single cells and cultured as previously described (Nadal et al. 1998). Briefly, islets were disaggregated into single cells with trypsin. Cells were then centrifuged, resuspended in RPMI 1640 culture medium supplemented with 10% fetal calf serum, 200 U/mL penicillin, 0.2 mg/mL streptomycin, 2 mM L-glutamine, and 11 mM glucose and plated on poly-L-lysine-coated glass coverslips. Cells were kept at 37[degrees]C in a humidified atmosphere of 95% [O.sub.2] and 5% C[O.sub.2] for 24 hr.
Estradiol-HRP binding assay. The E-HRP binding assay was performed as previously described (Nadal et al. 2000; Ropero et al. 2002). Briefly, after 24 hr in culture, cells were gently fixed to avoid permeabilization and exposed overnight to 4.5 [micro]g/mL E-HRP plus the testing reagent at 4[degrees]C. This was the appropriate concentration of E-HRP to obtain a suitable labeling of the cell membrane. E-HRP binding was visualized by the precipitate formed when reacting with 3,3'-diaminobenzidine tetrahydrochloride (DAB) in the presence of urea hydrogen peroxide and Co[Cl.sub.2] for 15 min [SIGMA FAST DAB with metal enhancer tablet set (Co-DAB)]. The light absorbed by the precipitate was measured; the lower the percentage of absorbed light, the higher the competition for an E-HRP binding site. The quantity of E-HRP bound is expressed as the percentage of absorbed light compared with the control condition. In order to obtain an appropriate staining background, incubation and developmental procedures with peroxidase were performed in identical conditions. All of the chemicals tested for competition with EHRP were used in a concentration 300-fold higher than that of E-HRP.
Immunoeytochemistry. [alpha]-Cells were identified using antiglucagon antibody labeling. After staining with E-HRP and Co-DAB, the cells were permeabilized with 1% Triton X-100 for 2 min, and immunocytochemistry was performed as previously described (Ropero et al. 2002).
Results
Effect of EDCs and [E.sub.2] on low-glucose--induced [[[Ca.sup.2+]].sub.i] oscillations. To investigate the effect of [E.sub.2] and EDCs in pancreatic [alpha]-cells, [[[Ca.sup.2+]].sub.i] recordings were obtained from intact islets of Langerhans (Figure 1A) loaded with the fluorescent calcium-sensitive dye Fluo-3 and imaged using laser scanning confocal microscopy (Figure 1B). Although only the periphery of the islet is loaded, as previously described (Nadal et al. 1999; Ropero et al. 2002), all the different types of cells within the islet are represented and can be easily identified by their [[[Ca.sup.2+]].sub.i] response to glucose (Nadal et al. 1999; Quesada et al. 1999). Pancreatic [alpha]-cells are distinguished by their particular oscillatory [[[Ca.sup.2+]].sub.i] pattern in the absence of glucose, which diminishes as the glucose concentration is increased (Figure 1C).
[FIGURE 1 OMITTED]
Typical [alpha]-cells presented a [[[Ca.sup.2+]].sub.i] oscillatory pattern in the absence of glucose, which was completely suppressed by 1 nM BPA in almost 50% (20 of 41) of the cells (Figure 2A,D) and by 1 nM DES in 31% of the cells (14 of 45 cells; Figure 2B,D). In all the remaining cells, both EDCs largely reduced the frequency of [[[Ca.sup.2+]].sub.i] oscillations (Figure 2E). To calculate the average effect on the frequency of [[[Ca.sup.2+]].sub.i] (Figure 2E), an average of all cells was performed. The pesticide 1,1,1-trichloro-2- [o-chlorophenyl]-2,2 [p-chlorophenyl] ethane (o,p'-DDT), was less active, producing a complete blockade in only 7% (3 of 41) of the cells tested (Figure 2C,D). The onset of the effect of BPA and DES was very rapid, and the decrease of the frequency of [[[Ca.sup.2+]].sub.i] oscillations was similar to the one obtained with [E.sub.2] and the membrane-impermeable E-HRP (Figure 2E,F). We used a concentration of E-HRP that was equivalent to the molecules of [E.sub.2]. Because the effect was present at a concentration equivalent to 1 nM [E.sub.2], the effect should be produced by the conjugated form; if free 17[beta]-[E.sub.2] was present, it would be in a much lower concentration. The effect of 0,p'-DDT was significantly smaller than that of the other estrogenic compounds (Figure 2E,F). [E.sub.2] and EDC effects were irreversible after 20 rain in a solution of 0.5 mM glucose, as previously described for [E.sub.2] (Ropero et al. 2002). The speediness of the action and the fact that E-HRP mimics the actions of [E.sub.2], BPA, and DES indicate that these EDCs act through an [E.sub.2] binding site, probably located at the plasma membrane.
[FIGURE 2 OMITTED]
A common membrane-binding site is shared by [E.sub.2], BPA, and DES. To further demonstrate the implication of a membrane binding site, we used a binding assay method based on the interaction of E-HRP as a specific probe to detect [E.sub.2] binding sites at the plasma membrane of nonpermeabilized cells. E-HRP bound to the plasma membrane is developed using DAB; the DAB-based primary reaction product of peroxidase was used as an indication of the amount of E-HRP bound to the estrogen-binding sites. This primary reaction product is highly absorptive of laser light at 543 nm and can be easily visualized with high contrast by using transmission laser scanning microscopy (Nadal et al. 2000). This assay has been combined with immunocytochemistry against glucagon performed afterward to study specifically the characteristics of the [E.sub.2] binding site in glucagon-containing [alpha]-cells (Ropero et al. 2002). Figure 3A shows several E-HRP control cells after being developed with Co-DAB; two of them were identified as glucagon-containing [alpha]-cells by immunostaining (Figure 3D). These control cells presented a black staining that absorbed the light of 543 nM from the laser beam (Figure 3G).
[FIGURE 3 OMITTED]
When DES was added at the same time as E-HRP, it decreased the binding of E-HRP to its binding site at the plasma membrane, producing a reduced amount of precipitate. This can be easily visualized by a lower light absorption (Figure 3B,E), and it has been quantified in Figure 3G. Figure 3C shows the basal level when [alpha]-cells are incubated only with HRP. This background level has been subtracted in all experiments.
Figure 3G shows that BPA, DES, and [E.sub.2] bind to the same membrane-binding site. Remarkably, the pesticide o,p'-DDT, which was weaker in inducing the abolishment of [[[Ca.sup.2+]].sub.i] signals, presented a very low competition. This may indicate that the EDCs containing phenol groups, that is, DES and BPA, mimic the effects of 17[beta]-[E.sub.2], which also contains a phenolic A-ring in its structure, by binding at the same membrane-binding site. However, o,p'-DDT, which contains no hydroxylated moieties in its structure, was less potent.
To characterize whether the membrane-binding site is an ncmER, we tested whether actions of BPA, DES, and [E.sub.2] were modified by the pure antiestrogen ICI182,780, which inhibits ER-mediated effects. Figure 4 shows that the effects of BPA and DES were not modified by ICI182,780. In fact, BPA completely suppressed [[[Ca.sup.2+]].sub.i] oscillations in all the cells tested (n = 8, four islets), and DES blocked low-glucose-induced [[[Ca.sup.2+]].sub.i] oscillations in 10 of 13 cells (five islets), and in the remaining three cells it largely reduced the frequency of [[[Ca.sup.2+]].sub.i] oscillations (Figure 4C). ICI182,780 did not modify the frequency of low-glucose-induced [[[Ca.sup.2+]].sub.i] oscillations (Figure 4C): ICI182,780 controls ICI1-ICI3 range from 0.8/min to 1.4/min, well within the range described for 0.5 mM glucose (Figure 2E). This, along with the experiment described in Figures 2 and 3, strongly suggests that EDC effects are mediated by a membrane ER with a different pharmacologic profile than that of the classical ERs.
[FIGURE 4 OMITTED]
cGMP and PKG mediate rapid effects of BPA and [E.sub.2]. The experiments described to this point suggested that the actions of BPA and DES are mediated through the same receptor as is 17[beta]-[E.sub.2] in [alpha]-cells. For that reason, we used BPA as the paradigmatic EDC in this system to further analyze the molecular pathway involved. Because we have previously described that cGMP and PKG were implicated in the case of the natural hormone (Ropero et al. 2002), we sought to study their effects on the action of BPA. Application of 10 [micro]M 8-bromo-guanosine 3',5'-cyclic monophosphate (8Br-cGMP) mimicked the effects of BPA, DES, and [E.sub.2] on [[[Ca.sup.2+]].sub.i] oscillations (Figure 5A), as previously demonstrated (Ropero et al. 2002; Sugino et al. 2002). Given that the action of cGMP was probably mediated by PKG, we tested the action of 8Br-cGMP in cells pretreated with the membrane-permeable PKG inhibitor KT-5823 for 1 hr. Pretreatment with the PKG inhibitor did not have any effect on low-glucose-induced [[[Ca.sup.2+]].sub.i] oscillations (Figure 5C), but it almost completely blocked 8Br-cGMP action (Figure 5B,C).
[FIGURE 5 OMITTED]
When we used KT-5823 pretreated islets to test the effect of BPA, we found that the PKG inhibitor completely blocked BPA action (Figure 6A,B). Thus, KT-5823 does not alter [[[Ca.sup.2+]].sub.i] oscillations but prevents the suppression of low-glucose-induced [[[Ca.sup.2+]].sub.i] oscillations by BPA, indicating that BPA's effect is exerted by a cGMP/PKG-mediared mechanism, as has been demonstrated for the natural hormone 17[beta]-[E.sub.2] (Figure 6C).
[FIGURE 6 OMITTED]
Involvement of soluble guanylate cyclase and NOS in BPA and [E.sub.2] actions. In many cell types, increases in cGMP levels are due to the activation of soluble guanylate cyclase (GC) after NO generation by NOS. To test if this pathway was responsible for the BPA and [E.sub.2] actions described above, we started by testing the effect of the NO donor sodium nitroprusside (SNP). In cells that oscillate as usual in the presence of 0.5 mM glucose, SNP abolishes these [[[Ca.sup.2+]].sub.i] oscillations (Figure 7A,C). This effect resembles those produced by 8Br-cGMP and the estrogenic compounds BPA and [E.sub.2]. To check whether the NO effects on [[[Ca.sup.2+]].sub.i] oscillations were mediated by an increase in the intracellular levels of cGMP, we applied SNP after blocking GC activity with the selective inhibitor ODQ (Garthwaite et al. 1995). As shown in Figure 7, B and C, ODQ did not modify the average oscillatory frequency of [[[Ca.sup.2+]].sub.i] but completely blocked SNP action. Thus, blockade of GC activity prevented the inhibitory effect of SNP, suggesting that the action of NO is probably mediated by an increase in the intracellular levels of cGMP.
[FIGURE 7 OMITTED]
To prove that the cGMP/PKG-mediated actions of BPA and [E.sub.2] described in Figure 6 involved the upstream activation of a GC, we tested the effects of these compounds in ODQ-treated islets. In Figure 8, we demonstrate that the BPA and [E.sub.2] inhibitions of low-glucose-induced [[[Ca.sup.2+]].sub.i] oscillations are completely suppressed in the presence of ODQ. Therefore, we have demonstrated that BPA and [E.sub.2] actions involve the activation of a GC, probably by NO that generates cGMP, which in turns activates PKG.
[FIGURE 8 OMITTED]
To assess whether NOS is responsible for the generation of NO, we used the specific antagonist N-nitro-L-arginine methyl ester (L-NAME), which is known to block NOS activation in pancreatic islets (Akesson et al. 1999; Novelli et al. 2004; Salehi et al. 1996). As shown in Figure 9, L-NAME completely reversed the effect of BPA and [E.sub.2] (Figure 9A, C,E), demonstrating that NOS activation and the concomitant NO increase are involved in the rapid actions of EDCs and [E.sub.2] in pancreatic [alpha]-cells.
[FIGURE 9 OMITTED]
BPA and [E.sub.2] effects involve a G-protein-coupled receptor. To asses the role of G-proteins in the actions of EDC and [E.sub.2] on [[[Ca.sup.2+]].sub.i] signals in [alpha]-cells, we used pertussis toxin (PTX), an inhibitor of G[[alpha].sub.i] and G[[alpha].sub.o]. After a 4 hr preincubation with 100 ng/mL PTX, no inhibitory effect of BPA and [E.sub.2] was observed (Figure 10A). Figure 10B shows the effect of 1 nM BPA in an [alpha]-cell within an islet obtained from the same mouse and in the same conditions but in the absence of PTX. The results obtained from different experiments are summarized in Figure 10E. These results support the involvement of either a G[[alpha].sub.i]- or G[[alpha].sub.o]-coupled membrane receptor in the [[[Ca.sup.2+]].sub.i] regulation by BPA and [E.sub.2].
[FIGURE 10 OMITTED]
Discussion
The findings described in this article demonstrate that the endocrine disruptors BPA and DES imitate the circulating hormone 17[beta]-[E.sub.2] in suppressing low-glucose-induced [[[Ca.sup.2+]].sub.i] oscillations in pancreatic [alpha]-cells within intact islets of Langerhans. This rapid effect is observed in a preparation that is almost identical to the physiologic situation: intact islets of Langerhans used directly after their isolation. The [[[Ca.sup.2+]].sub.i] oscillatory pattern in [beta]-cells observed in vivo (Fernandez and Valdeolmillos 2000; Sanchez-Andres et al. 1995) are identical to those described with the present method (Nadal et al. 1999; Santos et al. 1991). We assume by these experiments performed in [beta]-cells that both preparations behave in a similar manner.
It has been demonstrated that an ncmER is involved in the rapid nongenomic effects of estrogens in pancreatic [alpha]- and [beta]-cells within intact islets of Langerhans (Nadal et al. 2000; Ropero et al. 2002). This ncmER is pharmacologically different from classical ERs because it is insensitive to antiestrogens such as tamoxifen and ICI182,780 (Nadal et al. 2004). Moreover, in pancreatic [beta]-cells, BPA and DES bind to ncmER at doses similar to those of 17[beta]-[E.sub.2], inducing the potentiation of [[[Ca.sup.2+]].sub.i] oscillations (Nadal et al. 2000). In this article, we have shown that a common binding site exists for BPA, DES, and [E.sub.2] in immunocytochemically identified [alpha]-cells. This binding site has a different pharmacologic profile than classical ERs: it binds BPA, DES, and [E.sub.2] at a similar dose. Moreover, the action of these agents is triggered by remarkably low concentrations, [10.sup.-9] M. However, BPA is considered a weaker estrogen about 1,000- to 2,000-fold less potent than 17[beta]-[E.sub.2] when interacting with classical ERs located in the cell interior (Krishnan et al. 1993; Kuiper et al. 1997). This result, along with the absence of effect of the pure anti-estrogen ICI182,780, strongly indicates an action via a receptor other than the classical ER. In addition, the ncmER involved in the regulation of [[[Ca.sup.2+]].sub.i] by 17[beta]-[E.sub.2] in [alpha]-cells uses a cGMP/PKG-mediated pathway, the same one demonstrated here for BPA. Therefore, the receptor involved is very likely to be the same ncmER responsible for the 17[beta]-[E.sub.2] actions previously described in [alpha]- and [beta]-cells (Nadal et al. 2000; Ropero et al. 2002).
In other types of cells [E.sub.2] induced rapid cGMP/PKG-mediated actions. Upstream of cGMP/PKG, NOS is activated, producing NO and activating a GC that in turn increases cGMP levels (Chambliss and Shaul 2002; Rosenfeld et al. 2000; Xia and Krukoff 2004). In the present study, the suppression of low-glucose-induced [[[Ca.sup.2+]].sub.i] oscillations by [E.sub.2] and BPA was reproduced by the NO donor SNP. This effect was completely blocked when the GC inhibitor ODQ was used, which indicates that NO is exerting its effect by activating a GC. When the GC inhibitor was used, [E.sub.2] and BPA had no effect on low-glucose-induced [[[Ca.sup.2+]].sub.i] oscillations. Moreover, when the NOS inhibitor L-NAME was applied, [E.sub.2] and BPA produced no effect on [[[Ca.sup.2+]].sub.i]. This demonstrates that the actions of [E.sub.2] and BPA are mediated by NOS activation, which produces NO and activates a GC. This is responsible for the increase in cGMP and the downstream activation of PKG that will likely block ion channels (Figure 11).
[FIGURE 11 OMITTED]
The results for the mechanism of action of [E.sub.2] and BPA discussed above reinforce the idea that environmental estrogens imitate 17[beta]-[E.sub.2] actions not only through the classical pathway but also via other alternative pathways that do not necessarily involve classical ERs (Nadal et al. 2005). Alternative effects are triggered by classical and nonclassical ERs located in the cytosol and the plasma membrane (Nadal et al. 2001). Two main molecular pathways implicated are Src/PI3-Kinase/Akt and Src/Ras/ERKs, which subsequently may activate NOS, as well as other enzymes (Alexaki et al. 2004; Cardona-Gomez et al. 2003; Guerra et al. 2004; Migliaccio et al. 2002; Viso-Leon et al. 2004). A number of studies have shown that both classical ERs associated with the plasma membrane and other unique membrane ERs activate G-proteins, initiating different signaling cascades that, in some cases, include the activation of NOS (Doolan and Harvey 2003; Qiu et al. 2003; Razandi et al. 1999; Wyckoff et al. 2001). In the present study, the suppression of low-glucose-induced [[[Ca.sup.2+]].sub.i] oscillations is blocked by the action of PTX, an inhibitor of G[[alpha].sub.i] and G[[alpha].sub.o] indicating that a G-protein-coupled receptor (GPCR) is involved. All known GPCRs have seven transmembrane-spanning domains, whose intracellular domains propagate the signal from the receptor to the G-protein. It is possible that the ncmER found in the endocrine pancreas may be a seven-transmembrane domain GPCR, as is the case with the orphan receptor GPR30, which behaves as an estrogen membrane receptor in breast cancer cells (Thomas et al. 2005), and the new progestin receptor discovered in teleosts (Zhu et al. 2003). Another possibility is that a receptor with a different structure from GPCRs is coupled to a classical GPCR that activates G[alpha], which has been proposed for ER-[alpha] in endothelial cells (Wyckoff et al. 2001).
There are no extensive studies of endocrine disruptors acting through the same alternative pathways as the natural hormone 17[beta]-[E.sub.2]. Nonetheless, evidence has accumulated in recent years in this direction (Nadal et al. 2005). The activation of ERKs (extracellular signal-regulated kinases) by several xeno-estrogens has been described in GH3/B6 pituitary tumor cells (Bulayeva and Watson 2004). Recently, BPA and other xenoestrogens have been shown to mediate [Ca.sup.2+] influx and prolactin release in GH3/B6 pituitary tumor cells at low concentrations (Wozniak et al. 2005). Evidence shows that BPA activates NO synthesis in endothelial cells (Noguchi et al. 2002), the release of dopamine in PC12 cells (Yoneda et al. 2003), caspase-3 in neurons (Negishi et al. 2003), and ERKs and protein kinase C in immune system cells (Canesi et al. 2004). Because all of these actions are blocked by the pure antiestrogen ICI182,780, a classical ER should be involved.
Other actions triggered by endocrine disruptors are not blocked by ICI182,780, and they probably do not involve classical ERs. These include rapid effects of environmental pollutants, such as 4-octylphenol, nonylphenol, and o,p'-DDT, that inhibit [Ca.sup.2+] channels in smooth muscle cells (Ruehlmann et al. 1998); the activation of the nuclear factor of activated T-cell (NF-AT) signaling pathways (Lee et al. 2004); and the activation of AP-1-mediated gene expression in cells that do not express classic ERs (Frigo et al. 2002). In human breast cancer cells, BPA modifies [Ca.sup.2+] signals in a rapid manner (Walsh et al. 2005). In pancreatic [beta]-cells, low concentrations of BPA activate the transcription factor CREB via a calcium-dependent mechanism that is activated by an ncmER (Quesada et al. 2002). CREB phosphorylation activated by BPA will bind to CRE (cAMP-responsive element) and in turn modulate DNA transcription. This suggests that the activation of steroid membrane receptors can produce cellular effects through genomic mechanisms.
There is a current debate regarding the concentration of EDCs needed to produce biologic effects in both humans and animals (Kaiser 2000; Welshons et al. 2003), especially because BPA is highly unstable in water and undergoes biodegradation to a less active compound (Staples et al. 1998; Zalko et al. 2003). This would account for the BPA half-life of 2 days estimated in rivers (Zalko et al. 2003). Despite this, drinking water still has traces of BPA (Takahashi and Oishi 2000), which have cellular effects (Hunt et al. 2003; Quesada et al. 2002; Welshons et al. 2003). In the endocrine pancreas, only nanomolar concentrations of BPA are required to modify the physiology of [alpha]- and [beta]-cells. Furthermore, low concentrations of EDCs are enough not only to produce nongenomic effects but also to affect genomic pathways (Frigo et al. 20,32; Quesada et al. 2002).
[Ca.sup.2+] signals control many cellular processes, including secretion and gene expression. In pancreatic [alpha]-cells, glucagon release is [Ca.sup.2+] dependent. In the absence of glucose, [alpha]-cells present an oscillatory [[[Ca.sup.2+]].sub.i] pattern that triggers glucagon secretion; this pattern is suppressed as the concentration of glucose is increased (Nadal et al. 1999), and a diminished glucagon secretion can be expected (Johansson et al. 1987). Unlike [beta]-cells, [alpha]-cells are not synchronized (Nadal et al. 1999). Therefore, [alpha]-cells are subject to their individual sensitvity to stimuli, including low glucose. This may explain the heterogeneity in response to 17[beta]-[E.sub.2] and environmental estrogens. Like for low glucose, some [alpha]-cells respond to estrogen and xenoestrogens with complete blockade of calcium oscillations, whereas other cells only decrease the frequency of such oscillations (Nadal et al. 1999). The reason may be different levels of ncmER or any of the steps along the signaling pathway responsible for this effect. The effect of BPA and DES we observed is similar to the one produced by glucose, the suppression of the [[[Ca.sup.2+]].sub.i] movements that, in turn, decrease glucagon release (Nadal et al. 1999).
A disruption of glucose signaling process in [alpha]-cells by endocrine disruptors may have important implications for normal physiology. Glucagon acts via a seven-transmembrane domain GPCR (Jelinek et al. 1993), which has been identified in multiple tissues including liver, brain, kidney, intestine, adipose tissue, and pancreas (Christophe 1996; Jiang and Zhang 2003). The main physiologic role of glucagon is to stimulate the hepatic glucose output, increasing glycemia. This provides the major counter-regulatory mechanism for insulin by maintaining the glucose homeostasis in blood. It also has an important effect in inducing lipolysis and the release of fatty acids from adipose tissue when blood glucose is low. In the present study, [E.sub.2], BPA, and DES all decrease [alpha]-cell signaling when glucose is low. This effect should diminish glucogenolysis as well as lipolysis and fatty acid release from adipocytes, contributing to a higher adiposity.
Therefore, the signaling pathway underlying the effects of EDCs described in this study reinforces the existence of a new scenario that explains some of the low-dose effects of EDCs: an action through new binding sites that rapidly activate different signaling cascades outside the cell nucleus. Furthermore, it describes the possibility that low doses of EDCs affect the normal physiology of the endocrine pancreas, altering the regulation of glucose and lipid metabolisms.
REFERENCES
Akesson B, Henningsson R, Salehi A, Lundquist I. 1999. Islet constitutive nitric oxide synthase and glucose regulation of insulin release in mice. J Endocrinol 163:39-48.
Alexaki VI, Charalampopoulos I, Kampa M, Vassalou H, Theodoropoulos P, Stathopoulos EN, et al. 2004. Estrogen exerts neuroprotection effects via membrane estrogen receptors and rapid Akt/NOS activation. FASEB J 18:1594-1596. doi:10.1996/fj.04-1495fje [Online 2 August 2094].
Berne RM, Levy MN. 1993. Physiology. St Louis:Mosby-Year Book, 867-871.
Berts A, Ball A, Gylfe E, Hellman B. 1996. Suppression of [Ca.sup.2+] oscillations in glucagon-producing alpha 2-cells by insulin/glucose and amino acids. Biochim Biophys Acta 1318:212-216.
Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. 1995. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect 103:608-612.
Bulayeva NN, Watson CS. 2064. Xenoestrogen-induced ERK-1 and ERK-2 activation via multiple membrane-initiated signaling pathways. Environ Health Perspect 112:1481-1487.
Canesi L, Lorusso LC, Ciacci C, Betti M, Zampini M, Gallo G. 2004. Environmental estrogens can affect the function of mussel hemocytes through rapid modulation of kinase pathways. Gen Comp Endocrinol 138:58-69.
Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. 2003. Interactions of estrogen and insulin-like growth factor-I in the brain: molecular mechanisms and functional implications. J Steroid Biochem Mol Endocrinol 83:211-217.
Chambliss KL, Shaul PW. 2002. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev 23:665-686.
Christophe J. 1996. Glucagon and its receptor in various tissues. Ann NY Acad Sci 805:31-43.
Colborn T, Dumanowski D, Myers JP. 1996. Our Stolen Future. New York:Dutton.
Doolan CM, Harvey BJ. 2003. A G[alpha]s protein-coupled membrane receptor, distinct from the classical oestrogen receptor, transduces rapid effects from oestradiol on [[[Ca.sup.2+]].sub.i] in female rat distal colon. Mol Cell Endocrinol 199:87-103.
Fernandez J, Valdeolmillos M. 2000. Synchronous glucose-dependent [[[Ca.sup.2+]].sub.i] oscillations in mouse pancreatic islet of Langerhans recorded in vivo. FEBS Lett 477:33-36.
Frigo DE, Burow ME, Mitchell KA, Chiang TC, McLachlan JA. 2002. DDT and its metabolites alter gene expression in human uterine cell lines through estrogen receptor-independent mechanisms. Environ Health Perspect 110:1239-1245.
Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. 1995. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol 48:184-188.
Gerich JE. 1997. Metabolic abnormalities in impaired glucose tolerance. Metabolism 46:40-43.
Gopel S, Zhang Q, Eliasson L, Ma XS, Galvanovskis J, Kanno T, et al. 2004. Capacitance measurements of exocytosis in mouse pancreatic [alpha]-, [beta]- and [delta]-cells within intact islets of Langerhans. J Physiol 556:711-726.
Gopel SO, Kanno T, Barg S, Weng XG, Gromada J, Rorsman P. 2000. Regulation of glucagon release in mouse [alpha]-cells by [K.sub.ATP] channels and inactivation of TTX-sensitive channels. J Physiol 528:509-520.
Gromada J, Bokvist K, Ding WG, Barg S, Buschard K, Renstrom E, et al. 1997a. Adrenaline stimulates glucagon secretion in pancreatic A-cells by increasing the [Ca.sup.2+] current and the number of granules close to the L-type [Ca.sup.2+] channels. J Gen Physiol 110:217-228.
Gromada J, Ding WG, Barg S, Renstrom E, Rorsman P. 1997b. Multisite regulation of insulin secretion by cAMP-increasing agonist: evidence that glucagon-like peptide 1 and glucagon act via distinct receptors. Pflugers Arch 434:515-524.
Grunfeld HT, Bonefeld-Jorgensen EC. 2004. Effect of in vitro estrogenic pesticides on human oestrogen receptor [alpha] and [beta] mRNA levels. Toxicol Lett 151:467-480.
Guerra B, Diaz M, Alonso R, Marin R. 2004. Plasma membrane estrogen receptor mediates neuroprotection against [beta]-amyloid toxicity through activation of Raf-1/MEK/ERK cascade in septal-derived cholinergic SN56 cells. J Neurochem 91:99-109.
Guillette LJ Jr, Pickford DB, Crain DA, Rooney AA, Percival HF. 1996. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen Camp Endocrinol 101:32-42.
Hayes T, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. 2002. Herbicides: feminization of male frogs in the wild. Nature 419:895-896.
Hunt PA, Koehler KE, Susiarjo M, Hedges CA, Ilagan A, Voigt RC, et al. 2003. Bisphenol-A exposure causes meiotic aneuploidy in the female mouse. Curr Biol 13:546-553.
Jelinek LJ, Lok S, Rosenberg GB, Smith RA, Grant FJ, Biggs S, et al. 1993. Expression, cloning and signaling properties of the rat glucagon receptor. Science 259:1614-1816.
Jiang G, Zhang BB. 2003. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab 284:E671-E678.
Johansson H, Gylfe E, Hellman B. 1987. The actions of arginine and glucose on glucagon secretion are mediated by opposite effects on cytoplasmic [Ca.sup.2+]. Biochem Biophys Res Commun 147:309-314.
Kaiser J. 2000. Endocrine disrupters. Panel cautiously confirms low-dose effects. Science 290:695-697.
Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. 1993. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 132:2279-2286.
Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. 1997. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138:863-870.
Lee MH, Kim E, Kim TS. 2004. Exposure to 4-tert-octylphenol, and environmentally persistent alkylphenol, enhances interleukin-4 production in T cells via NF-AT activation. Toxicol Appl Pharmacol 197:19-28.
Ma X, Zhang Y, Gromada J, Sewing S, Berggren PO, Buschard K, et al. 2005. Glucagon stimulates exocytosis in mouse and rat pancreatic [alpha]-cells by binding to glucagon receptors. Mol Endocrinol 19:198-212.
McLachlan JA. 2001. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev 22:319-341.
Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, et al. 2002. Sex steroid hormones act as growth factors. J Steroid Biochem Mol Dial 83:31-35.
Nadal A, Alonso-Magdalena P, Ripoll C, Fuentes E. 2005. Disentangling the molecular mechanisms of action of endogenous and environmental estrogens. Pflugers Arch 449:335-343.
Nadal A, Diaz M, Valverde MA. 2001. The estrogen trinity: membrane, cytosolic and nuclear effects. Physiology 16:251-255.
Nadal A, Quesada I, Soria B. 1999. Homologous and heterologous asynchronicity between identified [alpha], [beta]- and [delta]-cells within intact islet of Langerhans. J Physiol 517(pt 1):85-93.
Nadal A, Ropero AB, Fuentes E, Soria B, Ripoll C. 2004. Estrogen and xenoestrogen actions on endocrine pancreas: from ion channel modulation to activation of nuclear function. Steroids 69:531-536.
Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. 2000. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci USA 97:11603-11608.
Nadal A, Rovira JM, Laribi G, Leon-Quinto T, Andreu E, Ripoll C, et al. 1998. Rapid insulinotropic effect of 17beta-estradiol via a plasma membrane receptor. FASEB J 12:1341-1348.
Negishi I, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. 2003. Inhibition of staurosporine-induced neuronal cell death by bisphenol A and nonylphenol in primary cultured rat hippocampal and cortical neurons. Neurosci Lett 353:99-102.
Newbold RR. 2004. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol 199;142-150.
Noguchi S, Nakatsuka M, Asagiri K, Habara T, Takata M, Konishi H, et al. 2002. Bisphenol A stimulates NO synthesis through a non-genomic estrogen receptor-mediated mechanism in mouse endothelial cells. Toxicol Lett 135:95-101.
Novelli N, Pocai A, Lajoix AD, Deify P, Bezzi D, Marchetti P, et al. 2004. Alteration of [beta]-cell constitutive NO synthase activity is involved in the abnormal insulin response to arginine in a new rat model of type 2 diabetes. Mol Cell Endocrinol 219:77-82.
Opara EC, Atwater I, Go VL. 1988. Characterization and control of pulsatile secretion of insulin and glucagon. Pancreas 3:484-487.
Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, et al. 2003. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled receptor that activates protein kinase C. J Neurosci 23:9529-9540.
Quesada I, Fuentes E, Visa-Leon MC, Soria B, Ripoll C, Nadal A. 2002. Low doses of the endocrine disruptor bisphenol-A and the native hormone 17beta-estradiol rapidly activate transcription factor CREB. FASEB J 16:1671-1673.
Quesada I, Nadal A, Soria B. 1999. Different effects of tolbutamide and diazoxide in alpha, beta-, and delta-cells within intact islets of Langerhans. Diabetes 48:2390-2397.
Quesada I, Soria B. 2004. Intracellular location of [K.sub.ATP] channels and sulphonylurea receptors in the pancreatic [beta]-cell: new targets for oral antidiabetic agents. Curr Med Chem 11:2707-2716.
Razandi M, Pedram A, Greene GL, Levin ER. 1999. Cell membrane and nuclear estrogen receptors derive from single transcript: studies of ER[alpha] and ER[beta] expressed in CHO cells. Mol Endocrinol 13:307-319.
Ropero AB, Soda B, Nadal A. 2002. A nonclassical estrogen membrane receptor triggers rapid differential actions in the endocrine pancreas. Mol Endocrinol 16:497-505.
Rorsman P, Hellman 13. 1988. Voltage-activated currents in guinea pig pancreatic alpha 2 cells: evidence for [Ca.sup.2+]-dependent action potentials. J Gen Physiol 91:223-242.
Rosenfeld CR, White RE, Roy T, Cox BE. 2000. Calcium-activated potassium channels and nitric oxide coregulate estrogen-induced vasodilation. Am J Physiol 279:H319-H328.
Ruehlmann DO, Steinert JR, Valverde MA, Jacob R, Mann GE. 1998. Environmental estrogenic pollutants induce acute vascular relaxation by inhibiting L-type [Ca.sup.2+] channels in smooth muscle cells. FASEB J 12:613-619.
Salehi A, Calberg M, Henningson H, Lundquist I. 1996. Islet constitutive nitric oxide synthase: biochemical determination and regulatory function. Am J Physiol 270:C1634-C1641.
Sanchez-Andres JV, Gomis A, Valdeolmillos M. 1995. The electrical activity of mouse pancreatic beta-cells recorded in viva shows glucose-dependent oscillations. J Physiol 486(pt 1):223-228.
Santos RM, Rosario LM, Nadal A, Garcia-Sancho J, Soria B, Valdeolmillos M. 1991. Widespread synchronous [[[Ca.sup.2+]].sub.i] oscillations due to bursting electrical activity in single pancreatic islets. Pflugers Arch 418:417-422.
Sonnenschein C, Soto AM. 1998. An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol 65:143-150.
Soria B, Quesada I, Ropero AB, Pertusa JA, Martin F, Nadal A. 2004. Novel players in pancreatic islet signaling: from membrane receptors to nuclear channels. Diabetes 53:S86-S91.
Staples CA, Darn PB, Klecka GM, O'Block ST, Harris LR. 1998. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 36:2149-2173.
Sugino F, Ishikawa T, Nakada S, Kaneko Y, Yamamoto Y, Nakayama K. 2002. Inhibition by nitric oxide of [Ca.sup.2+] responses in rat pancreatic [alpha]-cells. Life Sci 71:81-89.
Sutter-Dub MT. 2002. Rapid non-genomic and genomic responses to progestogens, estrogens, and glucocorticoids in the endocrine pancreatic [beta]-cells, the adipocyte and other cell types. Steroids 67:77-93.
Takahashi D, Oishi S. 2000. Disposition of orally administered 2,2-bis(4-hydroxyphenyl)propane (bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ Health Perspect 108:931-935.
Thomas P, Pang Y, Filardo E J, Dang J. 2005. Identity of an estrogen membrane receptor coupled to a G-protein in human breast cancer cells. Endocrinology 146:624-632.
Unger RH, Orci L. 1981a. Glucagon and the A cell: physiology and pathophysiology (first two parts). N Engl J Med 304:1518-1524.
Unger HH, Orci L. 1981b. Glucagon and the A cell: physiology and pathophysiology (second of two parts). N Engl J Med 304:1575-1580.
Viso-Leon MC, Ripoll C, Nadal A. 2004. Oestradiol rapidly inhibits [Ca.sup.2+] signals in ciliary neurons through classical oestrogen receptors in cytoplasm. Pflugers Arch 449:33-41.
Walsh DE, Dockery P, Doolan CM. 2005. Estrogen receptor independent rapid non-genomic effects of environmental estrogens on [[[Ca.sup.2+]].sub.i] in human breast cancer cells. Mol Cell Endocrinol 230:23-30.
Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. 2003. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 111:994-1006.
Witorsch RJ. 2002. Low-dose in utero effects of xenoestrogens in mice and their relevance to humans: an analytical review of the literature. Food Chem Toxicol 40:905-912.
Wozniak AL, Bulayeva NN, Watson CS. 2005. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-[alpha]-mediated [Ca.sup.2+] fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect 113:431-439.
Wyckoff MH, Chambliss KL, Mineo C, Yuhanna IS, Mendelsohn ME, Mumby SM, Shaul PW. 2001. Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through G[alpha]i. J Biol Chem 276:27071-27076.
Xia Y, Krukoff TL. 2004. Estrogen induces nitric oxide production via activation of constitutive nitric oxide synthases in human neuroblastoma cells. Endocrinology 145:4550-4557.
Yoneda T, Hiroi T, Osada M, Asada A, Funae Y. 2093. Nongenomic modulation of dopamine release by bisphenol-A in PC12 cells. J Neurochem 87:1499-1508.
Zalko D, Soto AM, Dolo L, Dorio C, Rathahao E, Debrauwer L, et al. 2003. Biotransformations of bisphenol A in a mammalian model: answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice. Environ Health Perspect 111:309-320.
Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. 2003. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100:2231-2236.
Address correspondence to A. Nadal, Institute of Bioengineering, Miguel Hernandez University, San Juan Campus, Carretera Alicante-Valencia Km 87, Sant Joan d'Alacant 03550, Alicante, Spain. Telephone: 34-96-5919535. Fax: 34-96-5919547. E-mail: nadal@umh.es
We thank B. Fernandez (Generalitat Valenciana) for excellent technical assistance.
This study was supported by the Spanish Ministry of Education and Science grant BFI2002-01469 and Instituto de Salud Carlos III grants RCMN (C03/08) and 03/0178. P.A.-M. has a fellowship from the Ministry of Education and Science, O.L has a fellowship from the Spanish Ministry of Foreign Affairs, and A.B.R. has a fellowship from the Spanish Ministry of Science and Technology.
The authors declare they have no competing financial interests.
Received 3 February 2005; accepted 18 May 2005.
Paloma Alonso-Magdalena, Ouahiba Laribi, * Ana B. Ropero, ** Esther Fuentes, Cristina Ripoll, Bernat Soria, and Angel Nadal
Institute of Bioengineering, Miguel Hernandez University, Sant Joan d'Alacant, Alicante, Spain
* Current address: Life Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, California, USA. ** Current address: Division of Molecular Medicine, Department of Anesthesiology, David Geffen School of Medicine at UCLA, Los Angeles, California, USA.
COPYRIGHT 2005 National Institute of Environmental Health Sciences
COPYRIGHT 2005 Gale Group