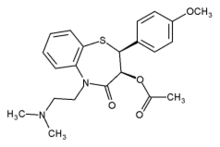
The authors explore the possibility of using extrusion--spheronization to develop a matrix-based, controlled-release formulation for diltiazem hydrochloride, a highly water-soluble drug. Various approaches involving modification of the formulation composition or process were adopted to achieve the target drug-release profile.
During the past few decades, various types of oral controlled-release (CR) formulations have been developed to improve the clinical efficacy of drugs having short half lives as well as to increase patient compliance (1,2). These formulations are designed to deliver drugs at a predetermined rate over a wide range of conditions and durations of therapeutic treatments. Matrix-based systems, in which the drug is dispersed as a fine powder from a matrix of polymeric- and non-polymeric release modifying agents, are preferred CR drug delivery systems because they are easy to manufacture (3).
The process of extrusion-spheronization (ES), introduced by Reynolds (4) and Conine and Hadley (5), has become the method of choice for developing multiunit, pellet-based dosage forms. ES offers the technological advantage of providing multiparticulates with a spherical shape, good flow properties, low friabilities, and uniform packing characteristics. In addition to high drug load, the pellet offers the advantage of achieving modified drug release by incorporating release modifying agents such as ethylcellulose, acrylic polymers, chitosan, and glyceryl monostearate in the formulation (6-9). However, the number of formulation aids and release modifying excipients available for ES is very limited because of the properties needed for their wet mass. Pellets can be compressed to yield a single unit dosage form or can be coated to deliver a drug at a desired rate at the same site or various sites within the gastrointestinal tract.
The application of ES for the development of CR formulations for highly water-soluble drugs without involving a coating step in the manufacturing operation is an area of interest because the methodology combines the advantages of a CR matrix system with an ES technology. Hence, the objective of the present study was to use ES to develop a matrix-based CR formulation for diltiazem hydrochloride (DLT), a highly water-soluble Class 1 drug that releases the drug at a predetermined rate on the basis of pharmacokinetic principles. The drug has a saturation solubility of ~590 mg/mL in phosphate buffer pH 7.4 (10). Various approaches involving the modification of the formulation composition or process were adopted to achieve the target drug-release profile. To test the effectiveness of using various excipients for successful ES, batch yields were determined and pellets were evaluated for physical characteristics such as particle size and distribution, crushing strength, and density.
Excipients were screened for compatibility with DLT before their use. Drug-release mechanisms and kinetics were elucidated, and the stability of selected formulations under accelerated temperature and humidity conditions were established.
Materials and methods
Materials. DLT (batch no. 0350200) was obtained from Cheminor Drugs Ltd. (India). The following chemicals were used in the formulation development:
* spheronization aid (SPA): microcrystalline cellulose (MCC), PH 101
* low-melting point hydrophobic agents: magnesium stearate, glyceryl monostearate, glyceryl palmitostearate, and bees wax
* water-swellable polymers: hydroxypropyl methylcellulose (HPMC), low-substituted hydroxypropyl cellulose (L-HPC), and hydroxypropyl cellulose
* water-insoluble excipients: ethylcellulose
* binders: HPMC, polyvinyl pyrolidone (PVP), and Eudragit NE 30 D.
HPMC, MCC, magnesium stearate, and PVP were obtained from Panacea Biotech Ltd. (India). Glyceryl monostearate, ethyl-cellulose, lactose, and bees wax were purchased locally. Glyceryl palmitostearate and Eudragit NE 30D were obtained from Gattefosse (France) and Rohm Gmbh (Germany), respectively. L-HPC 11 and L-HPC 20 were obtained from Shin-Etsu (Japan). All materials were used as received.
Methods. Theoretical design of controlled drug-release profile. Using the superposition method described by Ritschel, a theoretical controlled drug-release profile for DLT was developed on the basis of desirable target blood concentrations and pharmacokinetic properties of the drug (11). The steady-state drug concentration in the blood was predicted by using the same method. The first-order drug-release rate ([R.sup.0]) was chosen by calculating the target release profile for dosage forms that would release the drug for a period of time ([t.sup.DEL]) shorter than the selected dosing interval ([tau] = 12 h).
Drug-excipient compatibility studies. Isothermal stress testing was performed on binary drug-excipient mixtures by exposing the mixtures to elevated temperatures and moisture levels to accelerate drug-excipient interaction, if any. Mixtures were analyzed using differential scanning calorimetry (DSC; Mettler-Toledo DSC [821.sup.e], Switzerland) and fourier transform infrared spectroscopy (FT-IR; Impact 410, Nicolet, Madison, WI).
Preparation of pellets. MCC is the most frequently used excipient to aid aqueous ES because it forms a plastic and cohesive mass upon wetting, which are the most desired characteristics for successful ES (12, 13). Hence, a basic pellet formulation consisting of the drug and MCC was used. Other excipients were incorporated in various combinations and proportions to modify the drug-release characteristics. The ES technique--including the uses of a rotating roller extruder (model 10, Caleva, UK) with a standard 1-mm screen and a spheronizer (model 120) with a 120-mm diameter (3x3 [mm.sup.2] pitch, 1-mm depth)--was used to prepare 50-g batches of pellets.
Weighed quantities of the raw materials passed through British standard sieves (BSS) no. 80 (Scientific Engineering Corp., India) and were dry mixed in a polyethylene bag for 10-15 min. The mixing time was optimized by content uniformity testing in initial batches (data not shown). The mixture was granulated using the granulation fluid to achieve the appropriate level of moisture content for extrusion and spheronization. The moisture content of the wet mass was determined using an infrared moisture balance (PM480 Delta Range integrated with an LP16 IR dryer and an LC45 printer, Mettler-Toledo) and reported as the percent weight loss of initial wet weight. The wet granulation mixture was extruded, and the extrudate was then immediately spheronized at a speed of 2950 rpm for 5 min to yield spherical pellets. The pellets were dried in glass-lined trays in a forced-circulation, hot-air oven (Narang Scientific Instruments, India) at 40[degrees]C for 10-12 h. The dried pellets were then filled and stored in screw-capped, high-density polyethylene (HDPE) bottles.
Some pellet formulations were prepared by dispersing the drug in molten, low-melting point hydrophobic agents as release retardants by heating them to their melting temperatures and then the drug was dispersed in it. The mixture then was cooled to room temperature under constant stirring to produce a solid mass that was either crushed by hand or milled with laboratory-scale multimill equipment (Gansons Ltd., India) to generate uniform sized powder and granules (passed through BSS sieve no. 30). To avoid product loss during preparation, low-melting point hydrophobic agents were prepared in slight excess so that the required quantity of powder (passed through BSS no. 80 mesh) was weighed and used for ES following the described procedure.
Compression of DLT pellets (size fraction passed through BSS no. 22 and retained on BSS no. 44 sieve; i.e., 355-1000 [micro]m size range, unless specified) was performed using a single-punch tablet press fitted with either 9- or 12.7-mm circular flat-faced punches. Weighed quantities of pellets, premixed with 0.2% w/w aerosil, were filled manually into the die and compacted manually. Tablets were stored in tightly closed HDPE bottles. Selected formulations were compressed to produce tablets with different combinations of hardness, thickness, diameter, and weight to determine the compression behavior of pellets and to characterize the effect of tablet dimensions on drug-release properties.
Characterization, Prepared pellets were evaluated for particle-size distribution, flow, and compressibility characteristics. In addition, pellets were subjected to both light and scanning electron microscopy (SEM). True density of pellets was determined using the solvent displacement method with benzene.
In vitro drug-release studies. Dissolution studies with DLT pellets or tablets (equivalent to 90 mg DLT; n = 3-6) were conducted using USP Apparatus I (rotating basket) dissolution method in a programmable dissolution tester USP Apparatus XXI/XXII (TDT-0P, Electrolab, India). Test specifications were as follows: USP I baskets rotating at 100 rpm with 900 mL of distilled water maintained at 37 [+ or -] 0.5[degrees]C as dissolution medium. The 5-mL samples were withdrawn at various time intervals during a 24-h time period, and the volume was immediately replenished with a fresh medium. Samples were filtered through a 0.45-[micro]m pore size filter (Minisart, Sartorius AG, Germany) and analyzed spectrophotometrically (DU640i, Beckman Coulter, Miami, FL) at 237 nm. Pellet shape, and any visible changes in shape, structure, and integrity during the dissolution study were recorded at the end of the experiment, Pellet residue remaining after the dissolution studies was dried at room temperature and preserved for SEM.
Release profiles comparison, Mathematical comparisons of dissolution curves from various formulations provide an opportunity to test the similarity between two dissolution profiles. Fit factors ([f.sub.1] and [f.sub.2]) proposed by Moore and Flanner were used for comparing the dissolution profiles (14). An [f.sub.2] value of [less than or equal to] 50 indicates similarity between two dissolution curves, whereas [f.sub.1] is used as an additional parameter to confirm the similarity when the value is [greater than or equal to] 15. In addition to fit factors, two model independent ratio parameters--mean dissolution time (MDT) and dissolution efficiency (DE)--were calculated for the DLT formulations for comparison of their drug-release profiles (15-17).
Release kinetics and mechanism. Drug-release data obtained from selected formulations were subjected to various drug-release models to establish mechanisms as well as kinetics of drug release as described previously (10). Criteria for selecting the most appropriate model were based on the highest coefficient of determination ([R.sup.2]), the smallest sum of squared residuals (SSQ), and Akaike information criteria (AIC) (18). [R.sup.2] was used to compare the fit of a formulation with various kinetic models, whereas, MC and SSQ in combination with [R.sup.2] values were used to compare the fit of various formulations with the same kinetic model. F statistics were used to check the occurrence of correlations by chance.
Stability studies. A freshly prepared batch of optimized DLT formulation was taken in 30-mL screw-capped HDPE bottles and stored in a refrigerator (Ultra, Godrej, India) at 8[degrees]C (control sample) and in a stability chamber (WTC, Binder, Tuttlingen, Germany) maintained at accelerated temperature and humidity conditions of 40[degrees]C and 75% RH. After four months of storage, samples from both stations were removed and analyzed for visual characteristics, crushing strength, drug content, and drug-release properties.
In vivo performance prediction of selected formulation. Drug-release parameters ([R.sup.0] and [t.sup.DEL]) obtained from in vitro data and the drug's pharmacokinetic properties were used for predicting blood drug concentrations-time profiles from single dose and at steady state from multiple dosing (11). The method of superposition was used for the steady-state concentration predictions. Values of [C.sub.ssmax] and [C.sub.ssmin], maximum and minimum steady-state concentration, respectively, were compared with the desired values calculated from a theoretically developed controlled drug-release profile.
The quality of CR formulations was evaluated by dosage form index (DI) (19). In addition, the forgiveness indexes (FI) and percent fluctuations were determined for the formulations (20). FI determines how much latitude the patient has in delaying the next dose, and the reduction in the post-dose duration of action ([D.sub.a]) ensues inferior forgiveness (21).
Results and discussion
Desired drug-release profiles. Desired drug-release profiles for twice-per-day DLT formulations were calculated from the drug's pharmacokinetic characteristics and desired steady-state drug concentrations (50-150 ng/mL) (22, 23). The desired drug-release parameters were 94-mg doses to be delivered at a first-order kinetic rate of 0.3309/h for a period of 6.95 h. The desired in vitro drug-release profile and predicted steady-state concentrations for dosing intervals of 12 and 24 h are shown in Figures 1a and 1b.
[FIGURE 1 OMITTED]
Drug-excipient compatibility. Stability and compatibility of DLT with four types of excipients--water-insoluble, water-soluble, low-melting point hydrophobic, and water-swellable cellulose polymers--were tested using DSC and Frill Enthalpy values ([DELTA]h normalized to weight) and the melting endotherm temperatures were obtained using DSC. For DLT-excipient mixtures, most of the cases were preserved and did not show any significant difference for the total study period of 60 days at 40[degrees]C and 75% RH. The exception was lactose, which was eliminated because it showed potential interaction during thermal stress testing. Similarly, an FTIR spectrum of DLT was preserved and no changes were detected in any of the tested DLT-excipient mixtures after storage at 40[degrees]C and 75% RH for 45 days. The results ruled out chemical incompatibilities in tested drug-excipient mixtures.
Formulation development. Various formulation strategies were adopted and tested with the objective of generating pellets with release characteristics and physical properties within acceptable limits. All strategies were derived from one or more of the following modifications in the formulation development (process and/or composition):
* use of release-controlling excipients such as low-melting point hydrophobic agents, water-swellable polymers, and water-insoluble excipients
* drug used as powder, dissolved in granulation fluid, or dispersed in a low-melting point hydrophobic agent
* use of various aqueous systems as binders for granulation (water, aqueous solution of binders, or aqueous dispersion of film-forming agent)
* drying temperature in combination with low-melting point hydrophobic agents
* compression of pellets to produce tablets.
These approaches and their effects on product quality, physical properties such as yield (total and product), crushing strength, densities (bulk, tapped, and true), pellet size, and drug-release properties are described later in this article. The strategies are separated into two sections: formulation composition modifications and process variable modifications. Ingredient compositions of the formulations prepared and their corresponding physical properties are presented in Tables I and II, respectively.
Formulation modification approaches. Use of low-melting point hydrophobic agents. Low-melting point hydrophobic agents such as magnesium stearate, glyceryl monostearate, and glyceryl palmitostearate were used as formulation components to make the matrix hydrophobic and retard the drug-release rate. The drug was mixed with dry-powder excipients and granulated using an aqueous polymeric binder solution. Because DLT is a highly water-soluble drug, granulation fluid and the moisture content of wet mass were expected to be very critical to the quality of the pellets.
On increasing magnesium stearate concentration from 5% to 11% w/w (formulation D1, D2, and D3), the amount of granulation fluid required for extrusion was decreased from 28.44 to 20.40 g. The moisture levels attained were highest in formulations with the lowest magnesium stearate concentration. This result was attributed to reduced concentrations of SPA in the formulations (these formulations had MCC as the only moisture-holding component) as magnesium stearate was incorporated at the expense of SPA. Total and pellet yield were gradually reduced with the increase in magnesium stearate concentration. Spherical pellets could be generated only with 5% w/w magnesium stearate. At higher concentrations, only dumb bell- or cylindrical rod-shaped pellets were produced. Mean pellet size increased from 653 to 733 pm and then decreased again to 624 [micro]m with 5, 8, and 11% w/w magnesium stearate, respectively (see Table III). No uniform trend in mean pellet size and size distribution existed with increasing magnesium stearate concentration. This result could be erroneous because for 8% and 11% w/w magnesium stearate levels, the product was a cylindrical rod shape and the size determination with sieve analysis could not have determined the actual dimensions and size distribution of product. The poor shape of the pellets was also reflected in high Carr index (CI) and Hausner ratio (HR) values, which increased as the concentrations of magnesium stearate increased. Therefore, it can be concluded that magnesium stearate had adverse effects on the spheronizing property of powdered mixtures. In addition, the poor aqueous affinity of magnesium stearate and its addition to formulations imparted a poor molding capacity to otherwise good spheronization characteristics of SPA.
To improve the physical characteristics, two other excipients belonging to the same category, glyceryl monostearate and glyceryl palmitostearate, also were tested. These excipients had better affinities to water as reflected by their relative ease of wet ting. Consistent with the results produced with magnesium stearate, the use of glyceryl monostearate also was characterized with lower moisture-level requirements of high concentrations. The moisture content of the wet mass required was drastically reduced (see Table I), which also affects the pellet yield adversely (see Table II). Unlike pellets made using magnesium stearate, spherical pellets could be generated even at a high concentration of glyceryl monostearate. Mean pellet size decreased with an increase in glyceryl monostearate content because of a shift in size distribution in favor of the lower size range, which was attributed to a lack of glyceryl monostearate to provide sufficient cohesiveness to wet mass. This resulted in a higher degree of fragmentation during spheronization. Poor densification during spheronization also was reflected in a de creased true density, bulk and tap density, and lower crushing strengths of pellets. The decrease in glyceryl monostearate content from 50% to 35% w/w (D13) but a higher binder concentration for granulation (HPMC as 10% w/w aqueous solution) required a similar amount of granulation fluid and resulted in decreased pellet yield (36% w/w). All other physical properties of the pellets (D13) were similar to those of D12 except mean pellet size. Excessive fines were produced as indicated by a decreased mean pellet size for D13. This result was caused by the insufficient moisture content of wet mass that was required to create desired levels of agglomeration during spheronization. However, with 10% w/w concentration of HPMC in water, a moisture level of 11% w/w in wet mass was sufficient for ES because the material had started becoming sticky and any further addition of granulation fluid would have hampered the ES process. D13 was modified by reducing GMS levels to 30% w/w and replacing binder HPMC with PVP (30% w/w aqueous solution) in granulation fluid (D14). This change not only improved the pellet size, but also produced harder pellets. All other physical properties were more or less unchanged.
Similar to glyceryl monostearate, mean pellet size, product yield, and crushing strength decreased as the concentration of glyceryl palmitostearate increased (see Table II). CI and HR values also increased which indicated adversely affected pellet shape and flow characteristics. These results were attributed to reduced plasticity of wet mass, thereby leading to poor agglomeration and production of fines during spheronization.
Drug release from all these formulations containing magnesium stearate, glyceryl monostearate, and glyceryl palmitostearate exhibited a high-burst release ([greater than or equal to] 40% within 1 h). The use of various concentrations of low-melting point hydrophobic agents in combination with various binders could not prevent the burst release. In addition, high-burst release also was ascribed to high aqueous solubility of drug, which led to the immediate release of the drug available on the pellet surface. In addition, the high surface area of the pellets further pronounced the burst effect because the release of drug from the pellet's surface enhances the permeation of aqueous dissolution media into the pellet matrix, which further results in rapid dissolution and diffusion of drug out of the pellets. The use of a larger size fraction of DLT pellets containing glyceryl palmitostearate did not improve burst release, thereby indicating that high drug solubility was the main reason for burst release.
Use of water-swellable polymers. Because the use of hydrophobic excipients did not produce the desired release profile, attempts were made to control the drug release by using water-swellable polymers as pellet matrix-forming agents. Water-swellable polymers are commonly used as matrix-forming excipients to achieve CR properties from drug delivery systems (24, 25). Upon contact with aqueous media, these polymers swell to form a gel by means of polymer chain relaxation mechanisms (26, 27). The drug release is controlled as a function of the swelling rate of the polymer, the diffusion rate of aqueous media into the swelling matrix, the diffusion rate of drug out of the swollen matrix, or the erosion rate of swollen matrix depending on the characteristics of the polymer used and the delivery system under study (24).
Four water-swellable polymers were incorporated as formulation components at the expense of SPA. Various granulation media--water, PVP in water, or an alcohol-water mixture--were used to prepare a wet mass. In these preparations, DLT was dissolved and dispersed in small amounts of granulation fluid and added to the dry-powder mixture comprising excipients. This procedure was conducted to avoid overwetting during wet massing because the presence of a highly water-soluble drug and water-swellable polymers in various grades and quantities were expected to have tackiness problems, thereby leading to high unpredictability in the amount of granulation fluid required for formulation. Subsequent to the addition of drug solution-dispersion, extra granulation fluid was added to achieve the desired moisture content in wet mass. The tendency of a powder mixture to form a tacky wet mass was at a minimum with L-HPC 11 and was found to increase with the use of higher viscosity grades of L-HPC 20 or polymers with known higher swelling properties (HPMC).
Because water-swellable polymers have enormous capabilities for holding water and swelling, a smaller amount of granulating fluid or water was required as the concentration of L-HPC 11 increased from 15% (D23) to 30% (D24). Pellet yield was reduced with a slight shift in size distribution in favor of a lower size, thereby reducing mean pellet size from 569 to 513 pm (see Table II). Crushing strength was almost unchanged. Pellets were of spherical shape and had good flow properties as suggested by their very low CI and HR values (see Table II). However, pellets showed high disintegration immediately on coming in contact with the aqueous medium in dissolution studies, releasing almost 80% of the drug within 15 min. Hence, a higher concentration of L-HPC 11 was used in combination with the polymer binder solution as aqueous granulation fluid (D25). Although total and pellet yields were increased in comparison with those of D23, the proportions of pellets generated in the desired size range were similar (see Table II). Pellet hardness increased from 5.83 to 6.79 N (see Table II). This result was attributed to the binder effect as well as to a higher concentration of L-HPC 11 in the D25 formulation. However, drug-release properties were not improved, and similar to the release of D23 and D24, high burst release was attained.
Because L-HPC 11 was ineffective in avoiding the burst release effect, a higher viscosity-grade polymer L-HPC 20 was used (D26). To improve pellet strength, the amount of granulation liquid was increased, which was anticipated to avoid immediate disintegration as observed with L-HPC 11. Total yields and pellet yields improved in comparison with those of D25, which had higher L-HPC 11 in combination with polymeric binder solution used as granulating fluid (see Table II). Though mean pellet size and crushing strength increased slightly in comparison with those of D25, the magnitude of burst effect was unaltered.
Because higher viscosity or higher molecular weight grades of water-swellable polymers form gels of higher viscosity and higher gel stability compared with gels formed by lower viscosity grades, a higher viscosity grade of a water-swellable polymer, hydroxypropyl cellulose was used in the formulation (28). Upon granulating the powder mixture with an aqueous polymeric binder, a high degree of tackiness was observed with hydroxypropyl cellulose. The batch (D27) could not be prepared because the wet mass produced a very sticky extrudate that clumped and could not be collected. An attempt was made with the ethanol-water mixture (1:1 v/v) to prepare a wet mass. With this combination of solvents, pellet size and yield were comparable with formulations containing L-HPC 11 and hydroxypropyl cellulose which could be generated but with reduced hardness (see Table II). Reduced crushing strength of pellets was caused by the use of organic solvents in the granulation liquid, because the use of organic liquid as the granulation fluid reduces the strength of pellets. Stronger pellets are produced as the mole fraction of water in a water organic solvent mixture is increased (29). Pellet density was higher than that generated for the L-HPC 20 formulation (D26), thus indicating a higher degree of densification achieved during spheronization.
>From these results, it was concluded that various grades and concentrations of water-swellable polymers--L-HPC 11, L-HPC 20, and hydroxypropyl cellulose--in combination with various granulation fluids could be used to produce pellets of acceptable quality and yields. However, the expected CR property could not be achieved. This finding was attributed to the rapid swelling of water-swellable polymers shown by all three grades to cause disintegration of the pellets rather than the formation of the gel structure. Hence, HPMC, a different high molecular weight, water-swellable polymer known to form a swollen gel matrix at a slower rate upon contact with aqueous fluids, was selected to test the suitability of water-swellable polymers to control drug release from a matrix-based pellet system.
Wet mass preparation with HPMC (D8) was extremely difficult because HPMC showed a high tendency to form a tacky mass. Smaller amounts of granulation fluid (smaller than those required for formulations containing L-HPC 11, LHPC 20, and hydroxypropyl cellulose) was required to prepare the wet mass. Mean pellet size increased because relatively larger fractions of pellets were in the 710-1000 pan range. However, almost 93% w/w of pellets were concentrated in the 355-1000 [micro]m size range. Pellet yield was relatively lower because of the loss of some of the wet mass that stuck to the extruder screen and spheronizer plate surface. The crushing strength of the pellets was very high (15 N), which was attributed to HPMC, a high viscosity-grade polymer that provides better cohesiveness even at lower moisture content. The dissolution studies showed that pellet disintegration was delayed to ~6-7 h. Although disintegration was not as rapid as those of the other water-swellable polymers, a burst release was still observed. Results suggested ineffectiveness of tested water-swellable polymers, when used alone, in controlling the drug release from multiunit matrix-based particulate systems.
Use of excipients and polymers in combination. From the results obtained using various low-melting point hydrophobic agents and water-swellable polymers, it was concluded that good quality pellets could be generated using these agents. However, burst release of DLT could not be controlled by means of matrix-based pellets. Hence, combination strategies were tried, in which various low-melting point hydrophobic agents were combined with water-insoluble excipients and water-swellable polymers. Because pellets containing HPMC showed some resistence to disintegration, it was selected for use in combinations with low melting hydrophobic agents. Similarly, ethylcellulose, a commonly used matrix-forming agent in tablets with excellent release retarding properties, was selected for combinations with low-melting point hydrophobic agents with the objective of retarding burst release. Studies with combinations of these excipients are discussed in the following sections.
Use of glyceryl palmitostearate and HPMC. Glyceryl palmitostearate and HPMC were combined in various proportions keeping SPA and DLT levels constant (D6 and D7). These results were compared with those from D5 and D8, which included glyceryl palmitostearate and HPMC alone, respectively. As the ratio of the two components increased in favor of HPMC, the amount of water required for wet massing also increased. This observation was unlike the results obtained with formulations containing glyceryl palmitostearate (D4 and D5) or LHPC 11 (D23 and D24), in which moisture requirements reduced with increasing proportions of glyceryl palmitostearate or L-HPC 11 in the formulation. The result was attributable to the decreasing content of SPA in these formulations (D4, D5, D23, and D24) that was replaced with glyceryl palmitostearate or L-HPC 11. All other physical properties were similar to the formulations containing glyceryl palmitostearate and L-HPC 11 alone. However, burst release was observed in both formulations even at various glyceryl palmitostearate and HPMC proportions.
Use of glyceryl monostearate and ethylcellulose. Glyceryl monostearate and ethylcellulose were used in combination along with an aqueous HPMC solution as the granulation fluid. Glyceryl monostearate and ethylcellulose were taken in various proportions and studied. As the ratio of glyceryl monostearate and ethylcellulose was changed from 2.5:1 (D15) to 1:2.5 w/w (D16) without changing the drug or SPA content, the amount of granulation fluid required for preparing the wet mass increased (see Table I). This result was expected because hydrophobic glyceryl monostearate was replaced with ethylcellulose, which has higher moisture holding capacity. Pellet yields were similar in both cases (~54% w/w for D15 and D16, respectively), but size distribution shifted in favor of the lower size which was reflected in the reduced mean pellet size (see Table II). However, drug-release properties were not improved because burst release was still observed. Hence, ethylcellulose concentration further increased at the expense of SPA to produce a glyceryl monostearate to ethylcellulose ratio of 2.5:2 (D17). In comparison with D15, D17 required a slightly smaller amount of granulation fluid, which is attributed to a decreased SPA content. There was no change in drug-release profiles. Another formulation of D18 was prepared, in which the glyceryl monostearate level was increased at the expense of DLT (decrease in drug content was replaced with SPA and glyceryl monostearate) keeping the ratio of glyceryl monostearate to ethylcellulose similar to that in D15 (i.e., 2.5:1 w/w). However, the drug-release pattern was unaltered, and significant burst release occurred.
An attempt was made to combine magnesium stearate, glyceryl monostearate, and ethylcellulose in one formulation with PVP as the granulation fluid (D33). Although pellet yield, mean pellet size, and other physical properties were within the acceptable range, drug release was characterized by burst release (see Table II).
Use of film-forming polymeric dispersion. An aqueous dispersion of film-forming polymer was used as the granulating agent because it was expected that the presence of a film-forming polymer in a matrix could help control the drug release. Solid content of aqueous dispersion added to the formulation was considered in the determination of the percent composition of various ingredients in the formulation (D19). To increase the solids content in the formulation, a higher amount of aqueous dispersion was used. The amount of aqueous dispersion was divided into three parts. The first part was mixed with a dry-powder mixture to generate a wet mass without forming lumps. The wet mass was stored at 45[degrees]C in a forced-air oven for 30 min (complete drying was avoided), followed by the addition of the second part of the granulation fluid. The mass was dried again under similar conditions and the third part was added. The wet mass thus produced was extruded and spheronized to generate pellets (D20). Pellet yields were higher for D20 as the moisture level attained was a slightly higher giving fraction of pellets in the desired size range. Mean pellet size was marginally increased for D20 in comparison with that of pellets from D19. Most of the pellets (~95% w/w) were concentrated in the 355-710 [micro]m size range in both cases. The two formulations reflected no improvement in controlling the drug release.
Process modification approaches. Dispersion of drug in molten LHA. Three low-melting point hydrophobic agents--glyceryl monostearate, glyceryl palmitostearate, and bees wax--were used. The drug was dispersed in the molten agents and the solid mass produced after cooling the mixture was powdered to produce a powder of DLT and low-melting point hydrophobic agents.
In the case of glyceryl monostearate (D29) and glyceryl palmitostearate (D30), DLT and low-melting point hydrophobic agents were taken in a ratio of 1:0.5 and 1:1.4 w/w, respectively. A solidified mass was crumbled by hand to yield powder. For bees wax (D31 and D32), the drug-to-bees wax ratio was 1:2 and 1:0.8 w/w, respectively and the solidified mass needed to be milled in a multimill to generate powder. Powdered mass passed through BSS no. 80 mesh was used for ES (see Table II).
As with other formulations, the moisture requirement was controlled by SPA levels so that lower SPA content in formulation resulted in less granulation fluid required to produce a wet mass. Pellet yields were satisfactory (33-47% w/w). In the case of bees wax, mean pellet size increased as the DLT-to-bees wax ratio shifted from 1:2 to 1:0.8 w/w because of an increased SPA level as bees wax was reduced. Despite good physical properties, the drug release from all these formulations could not be controlled. However, the drug was dispersed in a low-melting point hydrophobic agent but breaking the solid mass into fine powder may have generated free drug particles rather than coated or embedded drug particles in the agent. Changing the agent or using a higher low-melting point hydrophobic agent level did not improve drug-release properties further.
Effect of pellet drying temperature. To examine the effect of drying treatment on drug-release properties, the formulations containing the low-melting point hydrophobic agent and film-forming materials were dried at room and their melting temperatures. Previous studies show that such an approach can be used to yield matrix pellets (29-31). It was hypothesized that the drying of pellets at a slightly higher temperature than the melting point of matrix former would cause the molten matrix former to ill] the channels and capillaries of the matrix, thereby providing better control over the drug release. Different drying treatments to generate pellets from various formulations did not produce any positive effect in retarding the drug release (data not shown). Instead, an increased drug release was observed in all cases. This was attributed to a disrupted matrix structure caused by molten matrix former escaping out of the pellets. The result was reflected by the reduced true density of pellets caused by drying at an elevated temperature, suggesting an increased porosity of pellets subsequent to drying treatment.
Compression of pellets: drug-release studies for comparative evaluation. On the basis of the results obtained from pellet formulations of DLT, it was concluded that neither the formulation nor the process modification approaches were successful in achieving the desired drug-release profiles. In all the formulations, a prominent burst drug release was observed that could not be controlled by any of the approaches. Hence, pellets were compressed as an extension step in the process of the preparation of a controlled-release drug delivery system for DLT using ES. Pellets (355-1000 [micro]m size range) generated from various formulation compositions were compressed on a single-stroke tablet compression machine to produce tablets. Tablets were first evaluated for their drug-release properties followed by other evaluations for selected formulation. In general, as reflected by MDT, drug release from these tablets was better controlled than corresponding drug-release profiles produced from pellets (see Table III). Better control over drug release from tablets was ascribed to close packing of the pellets on compression, thus forming a matrix structure with limited porosity. Compression of pellets into single-unit tablets also reduced the surface area of drug releasing unit exposed to dissolution media, thereby avoiding the immediate burst release observed in the case of the pellets.
Comparative evaluation of various tablet formulations: formulation selection. Various model-independent parameters and first-order kinetic model parameters were determined from drug release profiles for comparing the release properties of various formulations (see Table III). Model-independent parameters showed that D4t, D7t, D21t, D22t, D29t, and D33t produced [f.sub.2] values [greater than or equal to] 50 when compared with the desired profile. D[E.sub.12 h] (area under the dissolution curve up to 12 h expressed as percentage of the area of rectangle described by desired 98.12% release in 12 h) as well as MDT values for these formulations were close to the desired values. Except for D22t, the remaining formulations showed [f.sub.1] values [less than or equal to] 15. These results suggested similarity of drug-release profiles from five tablet formulations to desired release profile. However, on fitting the dissolution data in a first-order kinetic equation, D4t, D7t, D21t, D22t, and D33t showed very poor correlation characterized by [R.sup.2], SSQ, AIC, and F-statistics. Only D29t fit well in the first-order kinetic model and exhibited drug-release rate (0.2757/h) close to the desired value (0.3311/h) with good correlation (high [R.sup.2], low SE, and SSQ, F-observed higher than F-critical and low AIC). The drug-release profile for D29t is shown in Figure 2. Hardness and friability of D29t also was determined. Diametrical crushing strength of D29t was 16.60 [+ or -] 0.46 kPa and loss in the weight of the tablets upon friability testing was [less than or equal to]0.5% w/w. Physical properties and yield of the pellet formulation (D29) also were satisfactory for a given batch size (see Table II). On the basis of these results, D29t was selected for the development as first-order kinetic CR tablets of DLT. The selected formulation was named DLT-TAB.
[FIGURE 2 OMITTED]
DLT-TAB. Compression behavior of pellets. D29 pellets were compressed to yield tablets of three hardness values. Tablet weight was kept constant (109- or 166-mg DLT content) and hardness was varied using several compression pressures to generate tablets of different thicknesses. The mean hardness values were 3.93, 6.77, and 16.60 kPa for 109-mg DLT tablets and 3.03, 8.60, and 20.20 kPa for 166-mg DLT tablets. The top and radial fracture surfaces of the tablets were observed under scanning electron microscopy (SEM) to examine the fragmentation behavior of the pellets under various compression pressures. The effect of compression on drug-release properties also was studied. Photomicrographs of 109-mg DLT tablets' top and fracture surfaces of various hardnesses were prepared from D29 pellets (see Figures 3a and 3b). Tablets with low (3.93 kPa), medium (6.77 kPa), and high (16.60 kPa) hardness values were referred to as LP, MP, and HP, respectively.
[FIGURE 3 OMITTED]
LP and MP tablets contained discrete pellets with dearly distinguishable boundaries. The separation distance between the pellets was smaller in the MP tablets, thus indicating closer packing of pellets compared with LP tablets. Although the pellets kept their integrity without any signs of fragmentation on compaction to generate LP and MP tablets, deformation of the pellets occured upon compression (Figure 3a).
In addition, fractures developed on the pellets' surfaces. Even though the fracture depth was restricted to the outer periphery of pellets, fractures were found predominantly at interpellet contact points propagating toward the center of the pellets (see Figure 3a). Pellet deformation in the MP tablets was higher than in the LP tablets. Upper as well as radial fracture surface of the HP tablets (DLT-TAB) did not show distinguishable pellet structures (see Figure 3b; photomicrographs A and B). However, pellets with clear boundaries were visible in fragments generated by radial crushing of the tablets (see Figure 3b). This finding indicates that the pellets existed in extremely close packing in HP tablets. In a study of the compression behavior of microcrystalline cellulose pellets, Johansson (32, 33) correlated tablet tensile strength with tablet air permeability and showed that lower permeability, corresponding to a more-closed intergranular structure, produced tablets of higher tensile strength.
Thus, a higher tensile strength of MP tablets indicated a closed intergranular pore structure. Pellet compression by deformation and a low incidence of fragmentation indicated that less energy was required for repositioning the primary particles in the pellets compared with the energy needed to separate the primary particles by formation of a fracture plane. Johansson described the difference between energy requirements for shearing and for fracturing resulting from of the stress on individual pellets during uniaxial compression in a die (32). Simultaneous stress application on pellets from several directions makes the fracturing of pellets relatively difficult. Hence, compression of the D29 pellets was caused primarily by deformation such that the pellets remained coherent units without significant flaws in their structures after compression.
Effect of pellet size. To study the effect of pellet size on the drug-release properties of the tablets, different size fractions of D29 pellets (retained on BSS no. 16, i.e., [greater than or equal to] 1000 [micro]m and 22/44 sieve, i.e, 355-710 [micro]m) were used to prepare 109- and 166-mg DLT tablets.
The drug release was similar for tablets prepared from two size fractions at both the tested dose levels of DLT, which is indicated by [f.sub.2] values [greater than or equal to]50 (see Figure 4). The similarity between the two size fractions of pellets in terms of drug-release properties suggest that they could be mixed and used for tablet preparation, thus increasing the usable pellet yield. Upon correlating this finding with SEM results, it was reaffirmed that even though discrete pellets within tablets were the actual drug-releasing units, the drug-release retardation effect was a function of the close packing of pellets irrespective of the pellet size used in the tablet preparation.
[FIGURE 4 OMITTED]
Effect of tablet structural integrity. The drug-release property of the DLT-TAB formulation was studied as a function of the structural integrity of the tablet. DLT-TAB was halved by diametrically compressing with a tablet hardness tester (TBH 20, Erweka) at 2.3 mm/s until it fractured and the radial fracture surfaces were exposed. Only halved tablets weighing [??] 749% of the respective intact tablet weights were used.
The results showed that breaking the DLT-TAB tablets in half produced an adverse effect on the drug-release properties (see Figure 5). Although the [f.sub.2] value was 51.55, which indicates a borderline similarity of drug release, there was a distinctly faster drug release observed in the halved tablets within the 2-6-h period of drug release in this study. This finding could be attributed to an increase in the exposed surface area in the halved tablets. However, drug release was similar to the halved and intact tablets during the initial and terminal portions of drug release. Absence of any burst release signified that pellets were packed closely after compression, thus preventing faster drug release in the early phase of the dissolution study, despite the increase in the halved tablets' exposed surface area.
[FIGURE 5 OMITTED]
SEM pictures show that tablet structure relaxes in an aqueous dissolution medium, thereby loosening the pellets and creating channels for drug release (see Figure 6). Distinct pellet boundaries within the tablet structures appear after exposure to dissolution media. Hence, after the initial relaxation of the tablet structure in a dissolution medium, a fast drug release was observed. In the later stages of time duration, the drug release leveled off with the release profile from intact tablets. A similar effect would also have been operative with intact DLTTAB tablets, however, the characteristic fast drug release in the middle part of the drug-release profile was expected to be less prominent with intact tablets because of the smaller drug-releasing surface area.
[FIGURE 6 OMITTED]
Drug-release kinetics and mechanism evaluation. DLT-TAB tablets remained intact after a 24-h drug-release time period without losing their shapes or sizes. However, cracks were observed on the surface of the tablets during the drug-release studies. From SEM studies, the boundaries of the pellets were clearly visible after the dissolution study on the outer tablet and the radial tablet fracture surfaces, thus indicating that the pellets were intact after compression. This finding is attributable to the closely packed pellets after compression under high pressure, which resulted in tablets without demarcated pellet boundaries. However, during drug-release studies, the tablet structure relaxes to loosen the whole matrix. As a result, the individual pellet boundary is shown in the tablet structure remaining after the drug-release studies were conducted (see Figure 6). Structural integrity as well as the retained shapes and sizes of the tablets during the drug-release study indicated that the drug-release mechanism was diffusion-and/or dissolution-controlled without any significant contribution of erosion. However, the controlled-release dissolution of the highly water-soluble drugs in an aqueous media was less probable, thereby leaving diffusion to be the drug-release mechanism.
Drug-release data from the DLT-TAB tablets study were applied to various kinetic models to elucidate the mechanisms and kinetics of drug release. The data fit well in the Baker-Lonsdale, Higuchi, and first-order kinetic models (see Table IV). The compatible fit of the first-order kinetic and Higuchi models indicated that the drug release is controlled by a concentration-dependent diffusion mechanism. This finding indicated that the tablets were a heterogenous matrix system in which the drug release occurred by means of a diffusion process through channels or capillaries in the matrix.
However, SEM studies indicated that pellets did not fragment under compression to produce a matrix system. Instead, pellets were closely packed with some deformation. Although the drug release occurred through the channels and pores present in between the closely packed pellets in the tablets by means of diffusion (in agreement with Higuchian kinetics), the ultimate drug-releasing units were discrete pellets. This result also was indicated by the close fit of the dissolution data to the Baker-Lonsdale model, which is developed from the Higuchi model and describes the drug release from spherical matrices. Hence, release retardation from the tablets, in comparison with the release from the pellets, was attributed primarily to the close packing of the pellets, which acted effectively to retard the penetration of dissolution media into the tablets and consequently to prolong the drug release. At the same time, the reduced surface area of the pellets (upon compression into tablets) exposed the dissolution media and prevented an immediate-burst release. The tablets' poor fit to the Hopfenberg model as well as the unaltered tablet shape and size during the dissolution study eliminated the possibility of erosion-controlled drug release. However, the high [R.sup.2] value generated using the Hixson-Crowell model was unexplainable because neither a diminished tablet dimension was observed nor a drug release was suspected to be limited by the drug's particle dissolution rate.
To characterize the diffusion kinetics, data were applied to the Peppas model and the diffusional exponent n was determined. The diffusion exponent n was 0.44, thereby indicating the mode of drug release to be Fickian diffusion (see Table IV) (34, 35). Pharmaceutical systems that show a good fit to the Peppas model also follow the Weibull model. DLT-TAB tablets' drug-release data were plotted according to the Weibull equation to characterize the shape of the curve. Because the shape parameter [less than or equal to] 1, the curve shape was expected to be parabolic with a higher initial slope and consistent with the exponential profile (see Table IV) (36).
Accelerated stability studies. Stability studies for DLT-TAB tablets were conducted under accelerated temperature and humidity conditions (40[degrees]C and 75% RH) for 4 months. Results of the drug assay, drug-release profiles, and hardness are presented in Table V.
No significant change was observed in the drug content of the tablets and was comparable with the drug content of the control samples. There were no signs of distinguishable changes in appearance, color, and texture after 4 months of storage under accelerated conditions. The drug-release profiles were similar because [f.sub.2] values were [greater than]50 for the control versus the test samples (see Table V). DLT-TAB tablets were stable for 4 months under accelerated stability test conditions.
Prediction of in vivo concentration-time profile from in vitro data. A steady-state drug concentration-time profile for the DLT-TAB tablets was predicted using its in vitro first-order kinetic release rate. Because the drug-release rate from the DLT-TAB tablets was slightly lower than the desired level (see Table VI), the predicted drug concentrations were also proportionately lower to the desired concentrations. DI and FI were calculated (see Table VI). Steady-state concentration predictions indicated that DLT-TAB tablets generated slightly lower FI values than those predicted from the desired drug-release profile because of a slower drug release from DLT-TAB tablets (see Table VI).
Conclusion
Extrusion-spheronization (ES), though an easy method of making multiunit particulate systems, offers very limited applicability because of the limited number of excipients that can be used for successful ES. Because the concept of matrix-based systems (single or multiunit) for controlled drug delivery is expected to provide time and cost advantages over other coating based systems, work was undertaken with the objective of identifying the feasibility of using ES for the development of matrix-based, controlled-release (CR) formulations for diltiazem hydrochloride (DLT), a highly water-soluble BCS Class I drug. A matrix-based multiunit pellet system for CR delivery of DLT could not be developed through various formulation and process modification strategies adopted in the present study. The high aqueous solubility of the drug and the large surface area of the pellets resulted in extensive burst release. Although pellet systems were characterized with high burst release, DLT tablets prepared by compression of the pellets gave the desired drug-release properties. In addition, the medium dose requirements made it necessary to incorporate drug in a higher proportion in the delivery system. This resulted in a higher fraction of drug available on the pellet surface, which contributed to a pronounced burst effect. The drug release from the DLT-TAB tablets took place by concentration-dependent diffusion process through channels or capillaries in a pellet matrix and those in between closely packed pellets. The drug release followed first-order kinetics and was controlled by the degree to which the pellets were closely packed. Results indicated that the DLT-TAB formulation was stable under accelerated temperature and humidity conditions (40[degrees]C and 75% RH) for 4 months. The in vitro drug-release profile from DLT-TAB tablets was predicted to produce steady-state drug concentrations within a desired range.
Acknowledgements
The authors thank the Council for Scientific and Industrial Research, New Delhi, India, for its senior research fellowship.
References
(1.) P. DeHaan and C.F. Lerk, "Oral Controlled Release Dosage Forms: A Review," Pharm. Weekbl. Sci. Ed. 6, 57-57 (1984).
(2.) V.H.K. Li and V.H.L. Lee, "Influence of Drug Properties and Routes of Drug Administration on the Design of Sustained and Controlled Release Systems," in Controlled Drug Delivery: Fundamentals and Applications, J.R Robinson and V.H.L. Lee, Eds. (Marcel Dekker, Inc., New York, NY 1987), pp. 3-94.
(3.) J.R. Cardinal, "Matrix Systems," in Medical Applications of Controlled Release: Classes of Systems, R.S. Langer and D.L. Wise, Eds. (CRC Press, Boca Raton, FL, 1984), pp. 41-67.
(4.) D.A. Reynolds, "A New Technique for the Production of Spherical Particles," Mfg. Chem. Aerosol News, 41, 40-43 (1970).
(5.) J.W. Conine and H.R. Hadley, "Preparation of Small Solid Pharmaceutical Spheres," Drug Cosmet. Ind. 106, 38-41 (1970).
(6.) C. Tapia, G. Buckton, and J.M. Newton, "Factors Influencing the Mechanism of Release from Sustained Release Matrix Pellets produced by Extrusion/Spheronization," Int. J. Pharm. 92 (2), 211-218 (1993).
(7.) S.R. Goskonda, G.A. Hileman, and S.M. Upadrashta, "Controlled Release Pellets by Extrusion-Spheronization," Int. J. Pharm. 111 (1), 89-97 (1994).
(8.) D. Blanque et al., "Some Factors Influencing the Formation and In Vitro Drug Release from Matrix Pellets Prepared by Extrusion/Spheronization," Int. J. Pharm. 119 (2), 203-211 (1995).
(9.) S.H. Neau, M.Y. Chow, and M.J. Durrani, "Fabrication and Characterization of Extruded and Spheronized Beads Containing Carbopol 974P, NF Resin," Int. J. Pharm. 131 (1), 47-55 (1996).
(10.) A. Sood and R. Panchagnula, "Drug Release Evaluation of Diltiazem CR Preparations," Int. J. Pharm. 175 (1), 95-107 (1998).
(11.) W.A. Ritschel, "Biopharmaceutic and Pharmacokinetic Aspects in the Design of Controlled Release Peroral Drug Delivery Systems," Drug Dev. Ind. Pharm. 15 (6, 7), 1073-1103 (1989).
(12.) R. Gandhi, C.L. Kaul, and R. Panchagnula, "Extrusion and Spheronisation in the Development of Oral Controlled Release Dosage Forms," Pharm. Sci. Technol. Today, 2 (4), 160-170 (1999).
(13.) P. Kleinebudde, "The Crystallite-Gel Model for Microcrystalline Cellulose in Wet Granulation, Extrusion, and Spheronization," Pharm. Res. 14 (6), 804-809 (1997).
(14.) J.W. Moore and H.H. Flanner, "Mathematical Comparison of Dissolution Profiles," Pharm. Technol. 20 (6), 64-74 (1996).
(15.) K.A. Khan and C.T. Rhodes, "Effect of Compression Pressure on the Dissolution Efficiency of Some Direct Compression Systems," Pharm. Acta. Helv. 47, 594-507 (1972).
(16.) K.A. Khan," The Concept of Dissolution Efficiency," J. Pharm. Pharmacol. 27, 48-49 (1975).
(17.) V. Pillay and R. Fassihi, "Evaluation and Comparison of Dissolution Data Derived from Different Modified Release Dosage Forms: An Alternative Method," J. Controlled Release 55 (1), 45-55 (1998).
(18.) P.V. Parab, C.K. Oh, and W.A. Ritschel, "Sustained Release from Precirola (Glycerol Palmitostearate) Matrix: Effect of Mannitol and Hydroxypropyl Methylcellulose on the Release of Theophylline," Drug Dev. Ind. Pharm. 12 (8, 9), 1309-1327 (1986).
(19.) M. Gibaldi and D. Perrier, in Pharmacokinetics (Marcel Dekker, Inc., New York, NY, 1982), pp. 182.
(20.) J.P. Skelly and W.H. Barr, "Regulatory Assessment," in Controlled Drug Delivery: Fundamentals and Applications, J.R. Robinson and V.H.L. Lee, Eds. (Marcel Dekker, Inc., New York, NY, 1987), pp. 293-334.
(21.) J. Urquhart, "How Much Compliance Is Enough?" Pharm. Res. 13 (1), 10-11 (1996).
(22.) L.Z. Benet, S. Oie, and I.B. Schwartz, "Design and Optimization of Dosage Regimens: Pharmacokinetic Data, "in Goodman and Gilman's The Pharmacological Basis of Therapeutics, J.G. Hardman and L.E. Limbird, Eds. (McGraw-Hill, New York, NY 1996), pp. 1707-1792.
(23.) W.A. Ritschel and G.L. Kearns, "Pharmacokinetic Parameters of Important Drugs," in Handbook of Basic Pharmacokinetics (American Pharmaceutical Association, Washington, DC, 1999), pp. 479-503.
(24.) K.V.R. Rao and K.P. Devi, "Swelling Controlled-Release Systems: Recent Developments and Applications," Int. J. Pharm. 48 (1-3), 1-13 (1988).
(25.) K.E. Uhrich et al., "Polymeric Systems for Controlled Drug Release" Chem. Rev. 99 (11), 3181-3198 (1999).
(26.) A.R. Rajabi-Siahboomi et al., "Imaging the Internal Structure of the Gel Layer in Hydrophilic Matrix Systems by NMR Microscopy," J. Pharm. Pharmacol. 44 (supp), 1062 (1992).
(27.) P. Colombo, "Swelling-controlled Release in Hydrogel Matrices for Oral Route," Adv. Drug Del. Rev. 11 (1-2), 37-57 (1993).
(28.) R. Chatlapalli and B.D. Rohera, "Physical Characterization of HPMC and HEC and Investigation of Their Use as Pelletization Aids," Int. J. Pharm. 161 (2), 179-193 (1998).
(29.) E.S. Ghali, G.H. Klinger, and J.B. Schwartz, "Thermal Treatment of Beads with Wax for Controlled Release," Drug Dev. Ind. Pharm. 15 (9), 1311-1328 (1989).
(30.) K.K. Peh and K.H. Yuen, "Development and In Vitro Evaluation of a Novel Multiparticulate Matrix Controlled Release Formulation of Theophylline," Drug Dev. Ind. Pharm. 21 (13), 1545-1555 (1995).
(31.) Y. Miyagawa et al., "Controlled-Release of Diclofenac Sodium from Wax Matrix Granule," Int. J. Pharm. 138 (2), 215-224 (1996).
(32.) B. Johansson et al., "Compression Behaviour and Compactability of Microcrystalline Cellulose Pellets in Relationship to Their Pore Structure and Mechanical Properties," Int. J. Pharm. 117 (1), 57-73 (1995).
(33.) B. Johansson and G. Alderborn, "Degree of Pellet Deformation during Compaction and Its Relationship to the Tensile Strength of Tablets Formed of Microcrystalline Cellulose Pellets," Int. J. Pharm. 132 (1, 2), 207-220 (1996).
(34.) N.A. Peppas, "Analysis of Fickian and non-Fickian Drug Release From Polymers," Pharm. Acta. Helv. 60, 110-111 (1985).
(35.) A. Sood and R. Panchagnula, "Role of Dissolution Studies in Controlled Release Drug Delivery Systems," S.T.P. Pharm. Sci. 9, 157-168 (1999).
(36.) F. Langenbucher, "Linearization of Dissolution Rate Curves by the Weibull Distribution," J. Pharm. Pharmacol. 24, 979-981 (1972).
(37.) F. Theeuwes and W. Bayne, "Dosage Form Index: An Objective Criterion for Evaluation and Controlled-Release Drug Delivery Systems," J. Pharm. Sci. 66 (10), 1388-1392 (1977).
Please rate this article.
On the Reader Service Card, circle a number:
342 Very useful and informative
343 Somewhat useful and informative
344 Not useful or informative Your feedback is important to us.
Anurag Sood, Yasvanth Ashokraj, and Ramesh Panchagnula *
* To whom all correspondence should be addressed.
Anurag Sood is a senior research scientist at Ranbaxy Research Laboratories, (Haryana, India). Yasvanth Ashokraj is a doctoral research scholar and Ramesh Panchagnula is a professor in the department of pharmaceutics at the National Institute of Pharmaceutical Education and Research, Sector 67, S.A.S. Nagar, Punjab, 160 062, India, tel. +91 172 2214682, fax +91 172 2214692, panchagnula@yahoo.com
COPYRIGHT 2004 Advanstar Communications, Inc.
COPYRIGHT 2004 Gale Group