U.S. Marine Corp and Army doctrine specifies a process for troops to remove the field protective mask in the aftermath of a chemical weapon attack. At the company/battery level, this procedure culminates in exposure of the respiratory system of selected troops to potential gas vapor. Commanders in the field rely on front-line corpsman and medics to provide lifesaving first aid in the event that toxic exposures take place. After this initial stabilization, casualties would be evacuated to an Echelon I medical facility, typically a Battalion Aid Station. The current tactical unmasking procedure, as specified in doctrine, is critically analyzed from a field medical perspective. Easy to implement recommendations are made, both to prevent lethal exposures and to better treat toxicity should prevention fail.
Introduction
Chemical weapons remain a credible threat to American Forces around the globe. In the years following Operation Desert Storm, U.S. ground troops have seen dramatic improvements in chemical protective equipment. The ability to don and conduct operations in chemical protective masks is well rehearsed by both Marine Corps and Army units during training and was battlefield tested during both wars against Iraq. No documented chemical attacks actually took place, however, and unmasking procedures were not definitively validated in combat. As a result of the common practice of tactical force dispersal on the battlefield, many units likely would conduct unmasking at the company/battery level. The safe transition from a chemically contaminated environment to a clean environment presents a potential vulnerability for our forces, especially at the small unit level (company/battery). U.S. Marine Corp and Army doctrine specifies a process for troops to remove the field protective mask in the aftermath of a chemical weapon attack. At the small unit level, this procedure culminates in exposure of the respiratory system of selected troops to potential gas vapor. Commanders in the field rely on front-line corpsman and medics to provide lifesaving first aid in the event that toxic exposures take place. After this initial stabilization, casualties would be evacuated to an Echelon I medical facility, typically a Battalion Aid Station (BAS) for further care.
Review of Current Procedure
The current U.S. Marine Corps and Army doctrine for unmasking contaminated personnel following a chemical weapon attack is presented in Table I. This process, which was used during Operation Iraqi Freedom, is problematic for several reasons. First, the utility is limited to those chemical agents that have rapid onset of symptoms after respiratory exposures. Such chemicals include the nerve agents, the cyanides ("blood agents"), chlorine, and lewisite. Although the mustards and the other pulmonary toxins (diphosgene, phosgene) can affect the respiratory system, symptoms are typically delayed for hours following even large dose exposures.1 second, from a psychological standpoint, a chemical attack, by itself, stresses welltrained troops, and the unmasking process adds anxiety. In this situation, leaders may find it difficult to give unmasking orders to junior troops, and individuals selected to unmask may have to be forced to do so. Such situations conceivably could lead to mistrust and reduced unit cohesion. Last, and most important, the preventable death of an American service member may result from strict adherence to the current procedure.
Although the sensitivity of selective unmasking to detect certain toxic substances is limited, the fact remains that after a chemical attack someone has to be first to remove his/her field protective mask. Given this reality, there are ways to improve this procedure and minimize battlefield casualties, both chemical and psychological.
Proposed Modifications
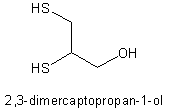
The current procedure can be improved by making four major modifications (Table II). First, adequate preparation for defense against a chemical attack must include prepackaging a medical unmasking kit. This kit should contain all supplies necessary for intravenous (IV)/intramuscular (IM) administration of antidotes, as well as all basic equipment needed for ventilatory support in a contaminated environment. Medications at the unmasking site should include IV atropine, IV pralidoxime, IV diazepam, FV sodium nitrite, IV sodium thiosulfate, IM dimercaprol, as well as either subcutaneous terbutaline or epinephrine. An airway kit including both oral and nasopharyngeal airways, a Resuscitation Device Individual Chemical, and a Laryngeal Mask Airway (LMA) is essential to sustain the severely toxic patient. The Resuscitation Device Individual Chemical is a self-inflating bag-valve mask containing the same replaceable filter used in the M40 protective mask. It allows rescuers to ventilate a patient with filtered ambient air or supplemental oxygen. Although not a definitive airway, the LMA has received a IIB recommendation as an advanced airway adjunct by the American Heart Association. Efficacy of the LMA approaches that of tracheal intubation.2 Use of the LMA is especially well suited for a chemical environment because it can be inserted blindly and, compared with trachéal intubation, requires only minimal training to achieve competency. In addition, the large distal ring prevents accidental insertion into the esophagus, reducing the likelihood of fatal errors. This feature is appreciated when one considers a corpsman/medic attempting to verify lung sounds through multiple layers of protective clothing while wearing a gas mask and hood. The contents of this unmasking kit are portable, lightweight, inexpensive, and have field utility beyond the contingencies listed in this article.
Second, if done appropriately, unmasking will take place on uncontaminated terrain. Therefore, the primary toxic threat to personnel removing their masks will come from off-gassing of liquids adherent to the unit's clothing and equipment. It becomes imperative to either decontaminate or discard these potential sources of secondary exposure before removing the mask. Vehicles should be parked downwind at a distance, since thorough decontamination is virtually impossible at the small unit level. Personal equipment should be cleaned or discarded. The field protective masks should then be cleaned of liquid. With the M40 mask, this can be accomplished by removing the eye covers and outer skin, changing the filter and wiping the remaining exposed surfaces with an M291/295 kit or similar product. Finally, after having minimized the potential for liquid agent exposure at the unmasking site, soiled personal protective overgarments should be discarded or exchanged.
Third, the unmasking protocol should be carried out with the assistance and supervision of corpsman or medics who are members of the unit equipped appropriately. Before exposing the troop's airway, PV access should be obtained either by cutting a flap in the new overgarment or by simply rolling up the uniform sleeve if the overgarment has already been discarded. For initial resuscitation, only small volumes of medication should be needed. Therefore, placement of a small bore (22gauge) over-the-needle catheter or a butterfly catheter with a saline lock would be adequate to facilitate rapid and effective delivery of life-sustaining antidotes before evacuation to an Echelon I BAS or similarly equipped facility.
Last, the current procedure of breaking the mask seal with eyes open while holding a breath makes good use of the eyes as the initial detection device, since virtually all chemical vapors have the potential to cause early irritation to the eyes. Although using the eye as an initial detection device may be uncomfortable, life-threatening effects would be unlikely and pain can be managed. For this reason, all unmasking should utilize this step even if the M256 kit fails to detect toxic vapors.
Discussion
Implementation of the proposed modifications should reduce the severity of any potential chemical poisoning and improve survivability following exposure. This is accomplished both by minimizing the dose of toxin and by maximizing immediate treatment. Of the potentially lethal chemical weapons, only the nerve agents, cyanogens, lewisite, and chlorine gas, would be expected to cause symptoms rapidly enough to be detected by elective unmasking procedures.
Nerve agents are the most lethal chemicals weapons and are relatively easy to employ, making them a major battlefield threat. Death from nerve agents results from respiratory failure secondary to suppression of central nervous system respiratory drive coupled with increases in airway secretions, bronchospasm, and respiratory muscle weakness.3 Atropine, pralidoxime, and diazepam reverse these effects. As with all medications, time to onset of action is largely dependent on the route of administration, with IV generally being the fastest method of achieving peak serum concentrations. Currently, however, most small units below the battalion level only carry these medications as IM autoinjectors. The survival of personnel exposed to lethal levels of nerve agent likely will depend on the ability of front-line medical personnel to deliver IV antidotes and filtered ventilations through a stable airway.
Compared with other chemical weapons, cyanogens have relatively low toxicity and are difficult to disperse over large areas. Cyanide does pose additional risks to troops, however, as an environmental hazard produced from burning plastics and synthetics. Additionally, cyanide is commonly found at industrial sites of strategic importance since large quantities are used in a variety of manufacturing processes.4 Exposure to a lethal concentration of cyanide results in death from anoxia at the cellular level via inhibition of cytochrome oxidase in the mitochondria and other mechanisms. Patients progress from loss of consciousness to apnea within minutes, and antidotes must be initiated rapidly. In fact, for large respiratory exposures, the life or death of the patient will depend on immediate treatment near the scene. It is unlikely that a person exposed to a lethal level of cyanide will live to reach a battlefield Echelon I BAS unless IV sodium thiosulfate, IV sodium nitrate, and ventilatory support are administered by front-line corpsman/medics before evacuation.
Lewisite is an arsenic-based vesicant that produces a rapid onset of pain after direct contact with skin, eyes, and mucous membranes. Actual vesicle formation, however, is typically delayed for hours. Additionally, delayed systemic symptoms may follow respiratory exposure to include "lewisite shock" and death via damage to multiple organ systems.5 Since vesication takes time, early death is unlikely. Over time, death may result from airway compromise due to obstruction by vesicles, edema, and inflammatory debris or as a result of systemic toxicity. Late systemic effects, possibly to include vesicle formation, can be minimized by early administration of dimercaprol given deep IM.
Chlorine gas, although no longer considered a major battlefield weapon, is commonly used for industrial processes. Similar to lewisite, chlorine causes rapid onset of painful burning symptoms after direct contact with eyes, skin, and mucous membranes.6 Inhalation of even low concentrations results in coughing and choking. The coughing is largely attributable to reactive bronchospasm. Most early deaths result from hypoxic respiratory failure during the first 24 hours after exposure.1 Asthmatic troops would be expected to be at high risk of early respiratory compromise. Because there are no effective antidotes, supportive treatment with bronchodilator therapy (terbutaline or epinephrine) and ventilatory support are all that is required for prehospital management.
If U.S. forces are exposed to an actual chemical agent attack in the future, the time spent by troops in their masks will be finite. Therefore, the safe transition from a chemically contaminated environment to a clean environment, by large numbers of forces, becomes an important contingency to plan. Understanding the utility of current doctrinal techniques for unmasking as well as the limits of current equipment and front-line medical skills is key to developing a good plan applicable at the company or battery level. By expending minimal resources in equipment and training, units can reduce potential fatalities, decrease psychological stress, increase the unit's confidence in its leaders, and improve overall mission accomplishment in the postattack phase of the chemical battlefield. The proposals here can serve as a framework to develop such a plan.
References
1. Urbanetti JS: Toxic inhalational injury. In: Textbook of Military Medicine: Medical Aspects of Chemical and Biological Warfare, pp 247-70. Washington, DC, Office of the Surgeon General, 1997.
2. ACLS Provider Manual, pp 32-33. American Heart Association, 2001.
3. Sidell FR: Nerve agents. In: Textbook of Military Medicine: Medical Aspects of Chemical and Biological Warfare, 129-79. Washington, DC, Office of the Surgeon General, 1997.
4. Baskin SI, Brewer TG: Cyanide Poisoning. In: Textbook of Military Medicine: Medical Aspects of Chemical and Biological Warfare, pp 271-86. Washington, DC, Office of the Surgeon General, 1997.
5. Sidell FR, Urbanett JS, Smith WJ, Hurst CG: Vesicants. In: Textbook of Military Medicine: Medical Aspects of Chemical and Biological Warfare, pp 197-228. Washington, DC, Office of the Surgeon General, 1997.
6. Bozeman WP, Dilbero D, Schauben JL: Biologic and chemical weapons of mass destruction. Emerg Med Clin North Am 2002; 20: 975-93.
Guarantor: LT David B. Rosenberg, MC USNR
Contributor: LT David B. Rosenberg, MC USNR
Battalion Surgeon, 5th Battalion. 10th Marines, 2nd Marine Division Camp. Lejeune, NC 28542.
Current address: General Medical Officer, Department of Family Medicine. Branch Health Clinic Oceana, Virginia Beach, VA 23460.
This manuscript was received for review in January 2004. The revised manuscript was accepted for publication in July 2004.
Copyright Association of Military Surgeons of the United States Jul 2005
Provided by ProQuest Information and Learning Company. All rights Reserved