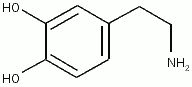
Inadequate splanchnic perfusion in the critically ill compromises the gut barrier leading to bacterial translocation, which is postulated to cause multiorgan dysfunction and failure. Inotropic agents such as dopexamine, dobutamine, and dopamine may have a role in increasing splanchnic perfusion, thereby protecting this area from further injury. This article examines the evidence for using these agents in patients with sepsis, postoperative trauma, and in those undergoing cardiac surgery and mechanical ventilation to increase gut perfusion and prevent multiple organ failure. Systemic effects of these agents differ from regional effects and must be considered when selecting therapy.
Key words: catecholamines; dopamine agents; sepsis; splanchnic circulation
Abbreviations: CPB = cardiopulmonary bypass; DA = dopamine; ICG = indocyanine green; PEEP = positive end-expiratory pressure; pHi = intramucosal pH; V[O.sub.2] = oxygen consumption
**********
Sepsis and the subsequent development of multiple organ failure are major problems in the critically ill. Microcirculatory disturbances are a hallmark of sepsis and septic shock. Decreased capillary flow and heterogenicity of microvascular perfusion have been implicated in the development of tissue hypoxia, cellular injury, and multiple organ failure. (1) Despite achieving normal hemodynamic values with conventional treatment, approximately 76% of septic patients die. (2)
The gut is an organ that is exquisitely sensitive to systemic cardiovascular and pulmonary disturbances. (3) As a physiologic response to a reduction in circulating volume, the blood is immediately shunted away from the splanchnic bed to more vital organs. (4) Inflammatory states, such as sepsis, are associated with a significant increase in gut and hepatic oxygen consumption (V[O.sub.2]). In addition, reperfusion efforts may further compound mucosal barrier dysfunction. (3)
Insults such as infection, trauma, bums, surgery, or cardiac compromise may increase the metabolic rate by 20%, resulting in higher V[O.sub.2]. Injury to the gut can also cause it to release additional inflammatory mediators. Tissue injury in sepsis is a consequence of progressive cellular death as a result of direct cytotoxic mediators that trigger apoptosis, or of cellular hypoxia developing as a result of abnormal oxygen delivery and utilization. (5)
It has been suggested that inadequate splanchnic perfusion leads to G1 mucosal ischemia, which in turn leads to increased permeability and bacterial/endotoxin translocation. It is postulated that this bacteria/endotoxin translocation may ultimately lead to multiple organ dysfunction. Adequate mucosal perfusion maintains the barrier function of the G1 tract. Loss of this barrier may allow bacteria and bacterial toxins to pass from the lumen of the gut into the systemic circulation, initiating or perpetuating septic events.
In patients with sepsis, the metabolic demand in the splanchnic region is elevated; therefore, therapeutic strategies to increase blood flow to the area have been suggested. Resuscitation and prompt correction of hypoxia and hypotension are necessary in the critically ill patient to help prevent splanchnic ischemia. Some experience suggests patient survival is improved if cardiac output and, hence, arterial oxygen delivery are maintained above normal. Because of differences in regional blood flow, the gut and liver may actually be inadequately perfused after these attempts. Both the GI tract and the liver can sustain reperfusion injuries despite normal systemic measures of adequate tissue oxygenation. In these cases, attention to regional blood flow should be considered.
There are a number of ways to increase blood flow to the gut and liver in the critically ill, including correcting hypovolemia and maintaining an adequate cardiac output. Various inotropic agents have vasodilatory properties and may also increase splanchnic blood flow.
DO DRUGS INCREASE SPLANCHNIC BLOOD FLOW?
Dopamine (DA) and selective DA receptor agonists have been shown previously to cause increases in blood flow to various organs. (6) We examine the evidence that supports the clinical application of these agents in increasing splanchnic blood flow with the aim of reducing multiple organ failure in the critically ill.
CONFOUNDING FACTORS IN THE STUDIES
Although several studies support the use of dopexamine, dobutamine, or DA for increasing splanchnic blood flow to reduce multiple organ failure, confounding factors must be taken into consideration. First, in all patients, volume resuscitation must be adequate. Additionally, patients may already be receiving other pressor or inotropic agents. Sometimes drags have a beneficial effect unrelated to blood flow, and different effects are seen at different dosages. The typical measures of splanchnic perfusion are listed in Table 1. Often studies that purport to measure blood flow actually measure something else. Occasionally, global measurements that seem adequate mask regional inadequacies. Additionally, restoration of blood flow to the gut still may not improve V[O.sub.2] and intramucosal pH (pHi) or reduce lactate production, and low pHi may reflect altered cellular metabolism, not low blood flow. Measurement of ileal blood flow is difficult in clinical practice. Many studies are performed in animals, because of the difficulty in measuring splanchnic blood flow in humans, but there are numerous differences between animals and people that may confound interpretations of the results.
CATECHOLAMINE RECEPTOR ACTION
Inotropic agents mediate vasoperfusion via dopamine receptors and other catecholamine receptors (Table 2). DA receptors, although five in number, are usually classified into two types. DA-1 receptors, represented by DA-1 and DA-5, stimulate adenyl cyclase, which provides a postsynaptic increase in protein kinase C activity. DA-1 receptors are found in the mesentery; coronary arteries; cerebral, gastric, and hepatic beds; and in the renal cortical tubules. DA-2 receptors, represented by DA-2, DA-3, and DA-4, inhibit adenyl cyclase. This action inhibits norepinephrine release from postganglionic sympathetic nerve endings. DA-2 receptors are found in the glomeruli and renal arteries. Their roles are as follows: (1) to mediate secretion of prolactin, (2) to inhibit aldosterone biosynthesis, and (3) to inhibit renin secretion from the kidney.
Dopexamine
Dopexamine, a DA analog developed for IV use in the treatment of heart failure and low cardiac output states, is not available for clinical use in the United States. (6) Dopexamine produces systemic vasodilation through stimulation of [[beta].sub.2]-adrenoceptors and peripheral DA-1 and prejunctional DA-2 receptors. It has a weakly positive inotropic activity that may be mediated via cardiac [[beta].sub.2]-adrenoceptors or by indirect [[beta].sub.1]-adrenoceptor activity, secondary to inhibition of neuronal catecholamine re-uptake. (6) Dopexamine has a potency relative to that of DA as a DA-1 agonist of 0.34, as a DA-2 agonist of 0.17, and as a [[beta].sub.1]-agonist of 0.1, but has 60 times the [[beta].sub.2]-agonist activity. (7) Dopexamine has no [alpha]-adrenergic receptor activity.
Early studies with dopexamine appeared to support its use in increasing splanchnic blood flow in the critically ill patient. Smithies and colleagues (8) examined 10 patients with sepsis syndrome, acute respiratory failure, and one other organ system failure. Patients were treated with a dopexamine infusion, maximum dose of 6 [micro]g/kg/min, to determine the clinical effects of the drug on systemic and splanchnic perfusion. Treatment with dopexamine increased gastric pHi from 7.21 to 7.28. Indocyanine green (ICG) clearance increased, demonstrating better liver perfusion while on dopexamine. The researchers concluded that dopexamine improved gastric pHi and thus splanchnic oxygenation. Maynard and colleagues (6) examined 25 patients with systemic inflammatory response syndrome receiving mechanical ventilation to assess the effect of low-dose dopexamine (1 [micro]g/kg/min) or DA (2.5 [micro]g/kg/min) on splanchnic blood flow. This group used gastric pHi, hepatic metabolism of lidocaine to monoethylglycinexylidide, and plasma disappearance rate of ICG as measurements. Results showed that dopexamine increased pHi from 7.25 to 7.35, increased ICG clearance from 7.6 to 11.3%, and increased monoethylglycinexylidide from 4 to 10.2 mg/mL. Treatment with DA showed no changes. The authors concluded dopexamine, but not DA, increased splanchnic blood flow.
More recently and in contrast, Meier-Hellmann and colleagues (7) found dobutamine and dopexamine to have no selective effect on splanchnic blood flow. They examined 12 patients with severe sepsis and treated them with volume loading and dobutamine infusion followed by increasing doses of dopexamine (0.5 to 4.0 [micro]g/kg/min). Splanchnic blood flow was measured by pHi and ICG clearance. Results showed that the splanchnic blood flow increased proportionately to the cardiac output; however, there was no selective effect of dopexamine on splanchnic blood flow. Also observed was a pHi decrease in a dose-dependent fashion suggesting a harmful effect on gastric mucosal perfusion. The authors suggest that the use of dopexamine as an additional catecholamine after treatment with dobutamine may preclude any further [beta]-receptor-mediated improvement in splanchnic circulation.
Using a different measurement technique, Temmesfeld-Wollbruck and colleagues (1) discovered that some areas of the splanchnic vasculature respond to dopexamine while others do not. Reflectance spectrophotometry for the assessment of mucosal hemoglobin oxygenation and concentration in the upper GI tract of septic patients demonstrated severe microcirculatory disturbances compared to healthy control subjects. Muller and colleagues (4) confirmed these findings in a placebo-controlled, randomized trial. Eighteen patients undergoing elective major abdominal surgery received either dopexamine, 1 [micro]g/kg/min, or 0.9% saline solution prior to measuring intestinal tissue P[O.sub.2] and gastric intramucosal PC[O.sub.2]. The dopexamine treatment increased small-bowel serosal P[O.sub.2] but not colon P[O.sub.2] or gastric mucosal PC[O.sub.2], demonstrating that there are variations in drug response by different parts of the gut.
There are some indications that the gut protection elicited by dopexamine is not fully explained by its effect on whole-body hemodynamics and oxygen transport variables alone. A mechanism of action for the ability of dopexamine to positively affect morbidity and mortality may be attributed to its anti-inflammatory properties. Byers and colleagues (3) studied the effects of dopexamine, 0.5 to 2.0 [micro]g/kg/min, on the incidence of acute inflammation in the stomach/duodenum of 38 high-risk patients undergoing abdominal surgery in a double-blind, randomized controlled trial. At 72 h postoperation, there were lower acute inflammatory changes and myeloperoxidase activity in the stomachs of patients in the dopexamine groups, demonstrating that dopexamine afforded significant histologic protection to the upper GI tract after surgery. Further evidence supporting the direct anti-inflammatory properties of dopexamine have been reported by Tighe and colleagues (2) in a porcine model of sepsis and by Schmidt and colleagues (9) in a rat videomicroscopy study. Another possible mechanism for explaining how dopexamine increases mucosal blood flow is the decreasing amplitude of flow motion as demonstrated by Madorin and colleagues (5) in a rat videomicroscopy study.
Dobutamine
Dobutamine is a catecholamine that can increase hepatic splanchnic blood flow but does not usually change or decrease pHi. Among the various adrenergic agents available today, dobutamine has been found to most consistently decrease mucosal-arterial PC[O.sub.2] difference and increase gastric mucosal blood flow. Dobutamine has been shown to improve both splanchnic oxygenation and gastric pHi in septic animals and in septic patients. (10)
Creteur and colleagues (10) analyzed the effects of short-term dobutamine infusion on the mucosal arterial PC[O.sub.2] difference and the hepatosplanchnic blood flow to determine if the adequacy of gut perfusion can be easily assessed by gastric tonometry in patients with severe sepsis. Dobutamine (5 [micro]g/kg/mL and 10 [micro]g/kg/mL) caused changes in the PC[O.sub.2] gap, which, in turn, revealed an increase in hepatosplanchnic perfusion. The proportion of blood flow (approximately 20%) being directed to the gut, however, was not substantially different.
Neviere and colleagues (11) also assessed whether dobutamine (5 [micro]g/kg/min) or DA (5 [micro]g/kg/min) infusion could increase gastric mucosal perfusion in a prospective randomized crossover trial in 10 patients with sepsis. Systemic hemodynamics, oxygen transport, and gastric perfusion were assessed by pHi and laser Doppler flowmetry. Gastric PC[O.sub.2] was decreased with dobutamine but remained the same with DA. Gastric blood flow was reduced in both groups.
DA
The splanchnic region is replete with vascular dopaminergic receptors. DA is a commonly used vasoactive agent known to cause splanchnic vasodilation, and possibly improving regional perfusion. In one of the first human studies of regional blood flow and oxygen transport, Ruokonen and colleagues (12) showed that, compared to 11 postoperative cardiac surgery patients, 10 patients with sepsis had higher splanchnic blood flow. This prospective, randomized, controlled trial examined systemic and regional hemodynamics and oxygen transport, measured by indirect calorimetry after treatment with norepinephrine or DA. They also examined splanchnic and leg blood flow, as measured by ICG infusion. In patients with sepsis, splanchnic blood flow was increased by both agents. DA increased splanchnic blood flow from 1.25 to 1.95 L/min/[m.sup.2] and as a percentage of cardiac output. Norepinephrine increased splanchnic blood flow from 0.93 to 1.25 L/min/[m.sup.2], but in an unpredictable fashion. This study demonstrated that septic shock is associated with major changes in regional blood flow and regional oxygen transport, which could not be predicted from systemic changes.
USE OF INOTROPIC SUPPORT IN CARDIAC SURGERY
Patients undergoing cardiac surgery with cardiopulmonary bypass (CPB) are at risk of acquiring systemic inflammatory response syndrome after the operation. (13) Preventing intestinal damage represents an important challenge because ischemic intestines have been shown to allow bacterial translocation and endotoxemia, which may be involved in the development of post-CPB inflammatory syndrome.
Berendes and colleagues (14) assessed the effects of dopexamine on renal function, splanchnic oxygenation, and systemic inflammation, and subsequent acute-phase response in otherwise healthy patients undergoing CPB surgery. Forty-four patients with a left ventricular ejection fraction [greater than or equal to] 0.5 were administered 0.5, 1.0, or 2.0 [micro]g/kg/ min dopexamine or placebo prior to, during, and after surgery. The dopexamine infusion increased systemic oxygen delivery, but hepatic venous oxygen saturation did not change; pHi decreased during and after CPB in all patients. Postoperative increases in interleukin-6 were lowest in the 2.0 [micro]g/kg/min group. C-reactive protein and serum amyloid A increases in the postoperative period were also less pronounced with dopexamine therapy. Creatinine clearance improved with all doses of dopexamine. While these results were promising, the authors concluded that routine use of continuous dopexamine infusion in otherwise healthy patients undergoing CPB was not justified.
Ensinger and colleagues (15) completed a randomized, controlled study of the effects of dobutamine on hepatosplanchnic blood flow, V[O.sub.2], glucose metabolism, and lactate and amino acid balance in 17 CPB patients. Variables were measured by ICG clearance and PC[O.sub.2] gap. Dobutamine increased the cardiac index, splanchnic blood flow, femoral blood flow, and the arterial-gastric mucosal PC[O.sub.2] gap. Treatment did not change gut V[O.sub.2]. These results suggest that, despite the predominantly [beta]-adrenergic activity of dobutamine, it does not increase the splanchnic metabolic demands even when splanchnic perfusion was significantly (0.8 to 1.0 L/min/[m.sup.2]) improved.
Human studies are difficult to perform because splanchnic blood flow is difficult to measure clinically; hence, animal studies provide the bulk of the available data. Bastien and colleagues (13) assessed the effects of CPB on splanchnic blood flow as measured by laser Doppler in three different splanchnic areas (gastric, jejunum, and ileum) of the rabbit and tested the potential of dopexamine to prevent CPB-induced decreased mesenteric blood flow. Splanchnic blood flow decreased significantly during CPB. Dopexamine improved jejunal and ileal blood flow, but not gastric blood flow. These results may help explain why pHi does not improve during CPB and also questions the use of pHi as an effective measurement of splanchnic blood flow.
A microsphere study was conducted in a pig model to determine whether low-dose DA infusion (5 [micro]g/min) during CPB selectively increases perfusion to the kidney, splanchnic organs, and brain at low (45 mm Hg) and high (90 mm Hg) perfusion pressures. (16) This randomized, crossover trial measured systemic perfusion, which was altered by adjusting pump flow rate. Investigators also examined cortical perfusion pressure, which increased from 178 mL/min/100 g at low perfusion pressure to 399 mL/min/100 g at high perfusion pressure. Treatment did not increase blood flow to the renal cortex, duodenum, jejunum, or ileum.
Positive End-Expiratory Pressure
Two animal studies from the same laboratory examined the effect of inotropic agents on the depression of mesenteric blood flow caused by positive end-expiratory pressure (PEEP) during mechanical ventilation. (17,18) Results showed that dopexamine was superior to DA in protecting mesenteric blood flow in the face of increasing levels of PEEP. Regardless, inotropic agents were not a replacement for adequate fluid loading to correct the depression in cardiac output and mesenteric blood flow associated with the use of mechanical ventilation and PEEP.
SUMMARY
Early identification of gut ischemia is an important step in preventing the development of multiple organ failure and its associated mortality. Inotropic agents have been suggested to play a role in correcting the problem of inadequate splanchnic blood flow. The evidence presented here demonstrates that dopexamine increases splanchnic blood flow and usually increases pHi in sepsis. Dopexamine may also have other beneficial effects on the gut, not clearly elucidated at this time. Further evidence of the positive effects of dopexamine are seen in investigations of its use in CPB, where dopexamine increases splanchnic blood flow. Dobutamine also increases splanchnic blood flow after CPB, also not as a fraction of cardiac output. In contrast, DA increases splanchnic blood flow in sepsis, but not after CPB.
REFERENCES
(1) Temmesfeld-Wollbruck B, Szalay A, Mayer K, et al. Abnormalities of gastric mucosal oxygenation in septic shock: partial responsiveness to dopexamine. Am J Respir Crit Care Med 1998; 157:1586-1592
(2) Tighe D, Moss R, Heywood G, et al. Goal-directed therapy with dopexamine, dobutamine, and volume expansion: effects of systemic oxygen transport on hepatic ultrastructure in porcine sepsis. Crit Care Med 1995; 23:1997-2007
(3) Byers RJ, Eddleston JM, Pearson RC, et al. Dopexamine reduces the incidence of acute inflammation in the gut mucosa after abdominal surgery in high-risk patients. Crit Care Med 1999; 27:1787-1793
(4) Muller M, Boldt J, Schindler E, et al. Effects of low-dose dopexamine on splanchnic oxygenation during major abdominal surgery. Crit Care Med 1999; 27:2389-2393
(5) Madorin WS, Martin CM, Sibbald WJ. Dopexamine attenuates flow motion in ileal mucosal arterioles in normotensive sepsis. Crit Care Med 1999; 27:394-400
(6) Maynard ND, Bihari DJ, Dalton RN, et al. Increasing splanchnic blood flow in the critically ill. Chest 1995; 108: 1648-1654
(7) Meier-Hellmann A, Bredle DL, Specht M, et al. Dopexamine increases splanchnic blood flow but decreases gastric mucosal pH in severe septic patients treated with dobutamine. Crit Care Med 1999; 27:2166-2171
(8) Smithies M, Yee TH, Jackson L, et al. Protecting the gut and the liver in the critically ill: effects of dopexamine. Crit Care Med 1994; 22:789-795
(9) Schmidt W, Hacker A, Gebhard MM, et al. Dopexamine attenuates endotoxin-induced microcirculatory changes in rat mesentery: role of [[beta].sub.2] adrenoceptors. Crit Care Med 1998; 26:1639-1645
(10) Creteur J, De Backer D, Vincent JL. A dobutamine test can disclose hepatosplanchnic hypoperfusion in septic patients. Am J Respir Crit Care Med 1999; 160:839-845
(11) Neviere R, Mathieu D, Chagnon J, et al. The contrasting effects of dobutamine and dopamine on gastric mucosal perfusion in septic patients. Am J Respir Crit Care Med 1996; 154:1684-1688
(12) Ruokonen E, Takala J, Kari A, et al. Regional blood flow and oxygen transport in septic shock. Crit Care Med 1993; 21:1296-1303
(13) Bastien O, Piriou V, Aouifi A, et al. Effects of dopexamine on blood flow in multiple splanchnic sites measured by laser Doppler velocimetry in rabbits undergoing cardiopulmonary bypass. Br J Anaesth 1999; 82:104-109
(14) Berendes E, Mollhoff T, Van Aken H, et al. Effects of dopexamine on creatinine clearance, systemic inflammation, and splanchnic oxygenation in patients undergoing coronary artery bypass grafting. Anesth Analg 1997; 84:950-957
(15) Ensinger H, Rantala A, Vogt J, et al. Effect of dobutamine on splanchnic carbohydrate metabolism and amino acid balance after cardiac surgery. Anesthesiology 1999; 91:1587-1595
(16) Mackay JH, Feerick AE, Woodson LC, et al. Increasing organ blood flow during cardiopulmonary bypass in pigs: comparison of dopamine and perfusion pressure. Crit Care Med 1995; 23:1090-1098
(17) Azar G, Love R, Choe E, et al. Neither dopamine nor dobutamine reverses the depression in mesenteric blood flow caused by positive end-expiratory pressure. J Trauma 1996; 40:679-687
(18) Steinberg S, Azar G, Love R. Dopexamine prevents depression of mesenteric blood flow caused by positive end-expiratory pressure in rats. Surgery 1996; 120:597-602
* From the Department of Anesthesia and Critical Care, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA.
Correspondence to: Alan Lisbon, MD, FCCP, Beth Israel Deaconess Medical Center, CC 470, 1 Deaconess Rd, Boston, MA 02215; e-mail: alisbon@caregroup.harvard.edu
COPYRIGHT 2003 American College of Chest Physicians
COPYRIGHT 2003 Gale Group