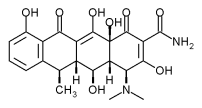
Abstract
This article will examine various clinical experiences with acne patients successfully treated with topical clindamycin 1% benzoyl peroxide 5% gel (Duac[R]) alone and in combination with other acne treatments. Clindamycin 1% benzoyl peroxide 5%, the only once-daily prescription topical aqueous gel combining a benzoyl peroxide and an antibiotic, has demonstrated excellent tolerability and efficacy and is stable when used concomitantly with other therapies. A regimen of topical clindamycin 1% benzoyl peroxide 5% gel, oral doxycycline hyclate (Doryx[R]), and adapalene gel (Differin[R]) seems especially advantageous from a theoretical and practical perspective. Improvement is noted by means of photographic, physician, and/or patient assessments at baseline and follow-up visits.
**********
Introduction
Acne has a multifactorial pathogenesis involving sebaceous gland hyperplasia, follicular hyperkeratinization, microbial proliferation, immune reactions, and inflammation. (1) The combination of high sebum production and follicular hyperkeratinization causes an obstruction in the sebaceous follicles. This blockage creates an environment for the proliferation of Propionibacterium acnes (P. acnes), (1) which releases chemotactic factors and proinflammatory mediators that may lead to inflammation. (2,3) P. acnes can also induce follicular keratinocytes to release interleukin-1, which leads to their proliferation and the formation of preclinical microcomedones. (4)
There are a variety of therapeutic agents available for the treatment of acne, including topical and systemic retinoids and antibiotics, and antibacterials, such as benzoyl peroxide (BP). The use of topical antibiotics, most commonly erythromycin, (5) is associated with the development of resistant strains of P. acnes, which decreases their effectiveness as monotherapy, but combination therapy with a topical antibiotic and BP may minimize the emergence of resistant strains. (6-9) The multifactorial nature of the pathogenesis of acne necessitates the use of a combination of different classes of therapeutic agents that act upon different areas of pathophysiology. For example, topical antibiotics reduce the population of P. acnes in the pilosebaceous duct, (10) while the addition of a topical retinoid normalizes desquamation of the follicular epithelium. (10,11) Furthermore, the combination of a retinoid plus BP combination product can promote faster resolution of existing acne lesions than the individual agents alone, and can also prevent the development of new lesions. (1)
Several combination products are available that combine either erythromycin or clindamycin with BP, which have demonstrated greater efficacy than either ingredient alone. (12-14) The efficacy and tolerability of 2 of these products, clindamycin 1% BP 5% gel and erythromycin 3% BP 5% gel (Benzamycin[R]) were assessed with BP 5% in a randomized, 10-week, multicenter, single-blind trial in 492 patients. (15) Results showed a nonsignificant trend for greater efficacy of clindamycin 1% BP 5% versus erythromycin 3% BP 5%, but overall, these combination agents were similarly efficacious. All 3 therapies demonstrated similar tolerability, with dry skin being the most frequent (< or = 7.3%) adverse event with all 3 therapies. These results mirror numerous other studies that have shown that all topical therapies cause some degree of irritation, especially BP, which can cause skin irritation and drying, and can cause contact allergy in up to 1% of patients. (10)
Although these combination products have demonstrated similar efficacy and tolerability, one drawback of erythromycin/BP combination products is that they require compounding by the pharmacist or patient, and require subsequent refrigeration. (16) Clindamycin 1% BP 5% gel, however, confers an advantage in that it is a uniform, aqueous gel that is manufactured under stringent US Food and Drug Administration (FDA) Good Manufacturing Practices and controls, which assure product consistency. (17) Since clindamycin 1% BP 5% is premixed, the manufacturing inconsistencies often encountered with human mixing and compounding do not occur. Additionally, the active ingredients have been shown to be stable for 60 days at 20[degrees] to 25[degrees]C (68[degrees]-77[degrees] F), so that neither the patient nor the physician ever need to store this gel in the refrigerator. (11,17) When kept at 2[degrees] to 8[degrees]C, the product is stable for 2 years. (17)
In addition to the stability and convenience of clindamycin 1% BP 5% gel, this product contains dimethicone and glycerin to moisturize and soften the skin. Dimethicone is an occlusive moisturizer that is hypoallergenic and lacks a strong odor. Occlusives condition the skin by impairing evaporation of water into the atmosphere. (18) Glycerin is a humectant moisturizer that attracts water from the viable skin layers to the stratum corneum. (18) An ideal formulation is one that combines occlusive and humectant ingredients to provide optimal rehydration, as products that contain only humectants actually increase transepidermal water loss. (18) The synergistic moisturizing properties of dimethicone and glycerin not only have the potential to enhance patient compliance and maximize the therapeutic benefits of clindamycin 1% BP 5% gel, but can preclude the need for supplemental moisturizers.
Efficacy and Tolerability of Clindamycin and BP 5% Gel The efficacy and tolerability of 1% clindamycin and 5% BP gel were reported in 2 11-week, double-blind investigations of 334 patients with acne vulgaris of the face. (14) Patients were randomized to receive once-daily treatment in the evening with either the combination gel (clindamycin phosphate equivalent to 1% clindamycin and 5% BP), clindamycin phosphate gel (equivalent to 1% clindamycin), BP gel (5%), or vehicle gel for 11 weeks.
In order to be included in the study, patients had to be between 13 and 30 years of age, with a minimum of 12 inflammatory lesions (papules and pustules) and 12 non-inflammatory lesions (open and closed comedones) with 3 or less nodulocystic lesions. (14) Patients were not permitted to use medicated shampoos or cleansers 1 week prior to the start of the study. In addition, because these studies were conducted for FDA approval, patients were prohibited from using acne treatments of any type, systemic or topical antibiotics or corticosteroids, or any medication that could interfere with the study results for 1 month, or oral isotretinoin for 6 months prior to study randomization and for the duration of the study.
[TABLE 2 OMITTED]
[FIGURE 1A OMITTED]
[FIGURE 1B OMITTED]
[FIGURE 2A OMITTED]
[FIGURE 2B OMITTED]
The mean number of inflammatory and non-inflammatory lesions at baseline and at 11 weeks, and the mean global improvement scores at week 11 are shown in Table 1. Results showed that 66% of the 1% clindamycin and 5% BP-treated group experienced a good or excellent global response at 11 weeks compared with 41% of the BP-treated patients, 36% of the clindamycin-treated patients, and 10% of the vehicle-treated patients (Table 2). (14) Global responses for all 3 active preparations were statistically significantly greater (P [less than or equal to] 0.001) than in the vehicle-treated group. The 1% clindamycin and 5% BP gel was also significantly superior (P [less than or equal to] 0.001) to the individual agents.
Each of the four gel preparations was well-tolerated with approximately 95% of patients rating their treatment as excellent. Most adverse events were mild, localized irritation with no statistically significant differences between the 3 active preparations versus the vehicle gel. (14) There was a significantly (P < 0.02) higher frequency of peeling in the 1% clindamycin and 5% BP- and BP-treated groups compared with the clindamycin-treated patients, and erythema occurred more frequently in the BP-treated group.
Because acne is usually treated with several different medications concurrently, the tolerability and efficacy of dual and even triple treatment regimens are of particular interest. In the following section, we describe and show several cases of acne vulgaris patients who successfully used clindamycin 1% BP 5% gel, alone and in combination with other acne treatments.
Clinical Experience with Clindamycin 1% BP 5% Gel
Patient 1 is a 26-year-old female with at least a 10-year history of acne vulgaris (Figure 1A). She had only used over-the-counter products and had never been seen by a dermatologist or other physician for acne. Upon examination, she had moderately severe grades 2 and 3 lesions of the right malar eminence, the medial third of the left cheek, and left malar eminence. Her forehead was essentially clear at presentation, with inflammatory lesions and scattered open and closed comedones on the chin. She was prescribed clindamycin 1% BP 5% gel to be applied once daily in the morning on her entire face and was instructed to use a non-irritating, non-comedogenic cleanser twice daily.
[FIGURE 3A OMITTED]
After 2 weeks of treatment, the patient's assessment of improvement was 50%. There was a marked decrease in the number of inflammatory papules and pustules of the anterior aspects of the cheeks, right malar eminence, the medial third of the left cheek, anterior chin, and forehead, and there was no evidence of new papules, pustules, or nodules (Figure 1B). At this time, she complained of slight drying. She was instructed to use a non-comedogenic moisturizer twice daily, and to continue using clindamycin 1% BP 5% gel as instructed.
[FIGURE 3B OMITTED]
After 6 weeks of treatment, there was even greater improvement (75%-80%). There was a decrease in all new inflammatory lesions on the forehead, cheeks, and chin, and no evidence of nodules. The majority of skin changes could be accounted for by postinflammatory erythema, and the patient was reassured that this would fade. There were one or two new inflammatory papules noted on the anterior third of the cheeks bilaterally, but nothing to warrant systemic treatment.
[FIGURE 4A OMITTED]
[FIGURE 4B OMITTED]
[FIGURE 4C OMITTED]
Patient 2 is an 18-year-old male with a 2-month flare of moderately severe grades 1, 2, and 3 acne (Figure 2A). Upon examination, he had inflammatory papules and pustules, and open and closed comedones of the forehead. He had tried 4 days of a sample of adapalene gel 0.1% and 2 months of an over-the-counter generic time-released BP/sulfur formulation without any effect. He was prescribed clindamycin 1% BP 5% gel once daily at night. He was also instructed to use a non-irritating, non-comedogenic cleanser twice daily.
After 6 weeks of treatment, the patient assessed improvement at 70%. There was a marked reduction in the number of inflammatory papules and pustules on the forehead (Figure 2B). His cheeks and chin were free of inflammatory and non-inflammatory lesions; there was postinflammatory erythema on the forehead, but he was reassured that this would fade over time.
Patient 3 is an 18-year-old male with inflammatory papules and pustules of the cheeks, neck, upper shoulders, and back (Figure 3A). He had been using ketoconazole (Nizoral[R]) tablets and adapalene gel 0.1%, but with little response. He was prescribed clindamycin 1% BP 5% gel to apply once daily at night to his face, neck, shoulders, and back. He was instructed to stop the adapalene gel 0.1% and to try a sample of ciclopirox 1% shampoo (Loprox[R]) to use on his scalp, face, shoulders, and back.
After 4 weeks of treatment, there was a dramatic improvement of the lesions on his face (Figure 3B). There were still minute, 1 to 2 mm papules and pustules (Pityrosporum folliculitis) on the upper back, shoulders, and cheeks. It was recommended that the patient start on itraconazole (Sporanox[R]), but he did not want to start taking pills at that time. Ciclopirox 1% shampoo was added to his clindamycin 1% BP 5% treatment regimen with continued success.
Patient 4 is a 17-year-old male who presented for follow-up for acne vulgaris. Upon examination, he had inflammatory papules and inflammatory comedones on the forehead and cheeks, as well as multiple open and closed comedones on the forehead, cheeks, and chin (Figure 4A). He had been using adapalene gel 0.1% once daily at night and a non-irritating, non-comedogenic cleanser twice daily. He was started on clindamycin 1% BP 5% gel to use sparingly every morning on his entire face and was instructed to continue using the adapalene gel 0.1% and the non-irritating, non-comedogenic cleanser.
After 4 weeks of treatment, there was a marked decrease in inflammatory lesions on the forehead, nose, cheeks, and chin. Open and closed comedones were still present on the forehead, cheeks, and chin (Figure 4B). After 6 more weeks of clindamycin 1% BP 5% and adapalene gel 0.1% combination therapy, there was a decrease in new lesions on the forehead, nose, cheeks, and chin (Figure 4C).
[FIGURE 5A OMITTED]
[FIGURE 5B OMITTED]
[FIGURE 6A OMITTED]
[FIGURE 6B OMITTED]
Patient 5 is a 23-year-old female with acne vulgaris who presented for follow-up. She had been using BP 6% gel (Triaz[R]) twice daily, adapalene gel 0.1% once daily at night, and a non-comedogenic moisturizer as needed for dryness, and a non-irritating, non-comedogenic cleanser. Upon examination, her forehead and cheeks were essentially clear, with one new inflammatory nodule on the right anterior chin, and resolving inflammatory lesions on both mandibles (Figure 5A). She was prescribed clindamycin 1% BP 5% gel to be applied once daily in the morning on her entire face. She was instructed to stop using the BP 6% gel, but to continue using the adapalene gel 0.1% once daily at night. After 6 weeks of clindamycin 1% BP 5% and adapalene gel 0.1% combination therapy, there was a decrease in new inflammatory lesions on the forehead, nose, cheeks, and chin with an especially marked improvement on the chin and anterior third of the mandibles (Figure 5B).
Patient 6 is a 17-year-old male with a 1- to 2-year history of acne vulgaris (Figure 6A). He was using tretinoin gel (Retin-A Micro[R]) every night for the previous 3 months. Prior to that, he had been using erythromycin 3% BP 5% without any effect. Upon examination, there were secondary excoriated inflammatory papules with open and closed comedones on the forehead, nose, cheeks, and chin. In addition, there was classic ice pick-like scarring on the medial mid-third of the cheeks. He was prescribed 100 mg of oral doxycycline hyclate to be taken once daily, and adapalene gel 0.1% to be applied once daily at night to his entire face. He was also instructed to use a non-irritating, non-comedogenic cleanser twice daily.
After 4 weeks of treatment, there was marked improvement with a substantial decrease in the number of inflammatory papules (70%-80%). There was still some small scarring on the cheeks as well as postinflammatory erythema of the cheeks. At this time, clindamycin 1% BP 5% gel, to be used once daily in the morning, was added to the treatment regimen. After 6 weeks of this treatment triad, there was a marked decrease in all new lesions with only minimal postinflammatory erythema of the cheeks (Figure 6B).
Discussion
These clinical reports demonstrate the tolerability and efficacy of clindamycin 1% BP 5% topical gel both as monotherapy and in combination with other acne vulgaris treatments. The combination of clindamycin 1% BP 5% gel provides the patient with a well-tolerated and efficacious therapeutic option. Clindamycin 1% has a mild comedolytic effect that reduces P. acnes and interleukin-1 production. (10) Topical antibiotics also provide some anti-inflammatory activity by suppressing leukocyte chemotaxis. (19) Both clindamycin 1% and BP 5% provide antibacterial activity, and the keratolytic action of BP may improve the penetration of clindamycin 1% into the skin. In patients 1 and 2 where clindamycin 1% BP 5% gel was used alone, there was a marked reduction in the number of inflammatory papules, pustules, and nodules in both patients, and a decrease in all new inflammatory lesions, with no evidence of nodules or cysts in patient 1. In patient 3, the daily regimen of clindamycin 1% BP 5% gel resulted in a dramatic improvement of inflammatory papules and pustules on the patient's face after 4 weeks of treatment.
Adapalene gel 0.1%, a second generation topical retinoid, has demonstrated a faster onset of action than tretinoin and is associated with significantly less skin irritation, (20) which may improve patient compliance. (10) The traditional approach of retinoid therapy has been to initiate treatment after an initial course of therapy with antibiotics with the primary goal of clearing comedones. (10) However, the increased tolerability of adapalene gel 0.1% provides the opportunity to prescribe this agent with an antimicrobial at the initiation of therapy for inflammatory acne. (10) In the present study, the regimen of adapalene gel 0.1% plus clindamycin 1% BP 5% gel used in patients 4 and 5 resulted in a marked decrease in inflammatory lesions and nodules on the forehead, nose, cheeks, and chin. After 6 more weeks of treatment, patient 4 experienced a decrease in new lesions on the forehead, nose, cheeks, and chin.
Oral antibiotics are highly efficacious in the treatment of inflammatory acne and are often added to a therapeutic regimen after lack of response to topical treatment. Finally, the triple combination of 100 mg oral doxycycline hyclate, adapalene gel 0.1%, and clindamycin 1% BP 5% gel prescribed for patient 6 was an especially effective treatment regimen. This patient was initially prescribed oral doxycycline hyclate once daily and adapalene gel 0.1% once daily at night to his entire face. After 4 weeks of treatment, there was a substantial decrease (70%-80%) in the number of inflammatory papules, but some ice pick-like scarring and postinflammatory scarring on the cheeks remained. Clindamycin 1% BP 5% gel, to be used once daily in the morning, was added to the treatment regimen. After 6 weeks of treatment with this therapeutic triad, the patient experienced a marked decrease in all new lesions with only minimal postinflammatory erythema of the cheeks.
The triple combination regimen of clindamycin 1% BP 5% gel, adapalene gel 0.1%, and doxycycline hyclate provides a treatment regimen with complementary mechanisms of action that remain stable in combination. Clindamycin has antimicrobial and anti-inflammatory effects and is mildly comedolytic, mechanisms that are enhanced by the antimicrobial and mildly keratolytic action of BP. Retinoids are very effective comedolytics and also may exert anti-inflammatory action. (21) Because topical retinoids normalize desquamation and reduce comedogenesis, the penetration of other topicals may be enhanced, which provides a more effective treatment of bacterial colonization. (10) These clinical reports help illustrate that the use of clindamycin 1% BP 5% gel as monotherapy and in combination reduces comedonal and inflammatory lesions for the acne patient. The synergism of these agents may contribute significantly to the faster onset of action and increased efficacy of combination therapy.
Disclosure: Dr. Bikowski receives honoraria and/or consultation fees from and/or may participate as a speaker for the following companies: Allergan, Aventis, Berlex, Biogen-Idec, CollaGenex, Dermik, Fujisawa, Galderma, GlaxoSmithKline, Healthpoint, Medicis, Novartis, Stiefel, 3M, and Warner Chilcott.
References
1. Gollnick H. Current concepts of the pathogenesis of acne: implications for drug treatment. Drugs. 2003;63:1579-1596.
2. Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158-3165.
3. Webster GF, Leyden JJ. Characterization of serum-independent polymorphonuclear leukocyte chemotactic factors produced by Propionibacterium acnes. Inflammation. 1980;4:261-269.
4. Guy R, Green MR, Kealey T. Modeling acne in vitro. J Invest Dermatol. 1996;106:176-182.
5. Leyden J, Kaidbey K, Levy SF. The combination formulation of clindamycin 1% plus benzoyl peroxide 5% versus 3 different formulations of topical clindamycin alone in the reduction of Propionibacterium acnes. An in vivo comparative study. Am J Clin Dermatol. 2001;2:263-266.
6. Eady EA, et al. Effects of benzoyl peroxide and erythromycin alone and in combination against antibiotic-sensitive and -resistant skin bacteria from acne patients. Br J Dermatol. 1994;131:331-336.
7. Eady EA, et al. The effect of acne treatment with a combination of benzoyl peroxide and erythromycin on skin carriage of erythromycin-resistant propionibacteria. Br J Dermatol. 1996;134:107-113.
8. Harkaway KS, et al. Antibiotic resistance patterns in coagulase-negative staphylococci after treatment with topical erythromycin, benzoyl peroxide, and combination therapy. Br J Dermatol. 1992;126:586-590.
9. Leyden JJ, Levy S. The development of antibiotic resistance in Propionibacterium acnes. Cutis. 2001;67:21-24.
10. Leyden JJ. A review of the use of combination therapies for the treatment of acne vulgaris. J Am Acad Dermatol. 2003;49:S200-S210.
11. Differin[R] (adapalene) Gel [package insert]. Fort Worth, Texas: Galderma Laboratories, LP. May, 2004.
12. Ellis CN, et al. Therapeutic studies with a new combination benzoyl peroxide/clindamycin topical gel in acne vulgaris. Cutis. 2001;67:13-20.
13. Chalker DK, et al. A double-blind study of the effectiveness of a 3% erythromycin and 5% benzoyl peroxide combination in the treatment of acne vulgaris. J Am Acad Dermatol. 1983;9:933-936.
14. Lookingbill DP, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997; 37:590-595.
15. Leyden JJ, et al. The efficacy and safety of a combination benzoyl peroxide/clindamycin topical gel compared with benzoyl peroxide alone and a benzoyl peroxide/erythromycin combination product. J Cutan Med Surg. 2001;5:37-42.
16. Russell JJ. Topical therapy for acne. Am Family Physician. 2000;61:357-366.
17. Fagundes DS, et al. Difference in the irritation potential and cosmetic acceptability of two combination topical acne gels: combined results of two comparative studies. Today's Therapeutic Trends. 2003;21:269-275.
18. Draelos ZD. Therapeutic moisturizers. Dermatol Clin. 2000;18:597-607.
19. Toyoda M, Morohashi M. An overview of topical antibiotics for acne treatment. Dermatology. 1998;196:130-134.
20. Cunliffe WJ, et al. A comparison of the efficacy and tolerability of adapalene 0.1% gel versus tretinoin 0.025% gel in patients with acne vulgaris: a meta-analysis of five randomized trials. Br J Dermatol. 1998;139:48-56.
21. Kligman AM. The growing importance of topical retinoids in clinical dermatology: a retrospective and prospective analysis. J Am Acad Dermatol. 1998;39:S2-S7.
Joseph B. Bikowski, MD
Bikowski Skin Care Center, Sewickley, PA
Address for Correspondence
Joseph B. Bikowski, MD
Bikowski Skin Care Center, Sewickley, PA
500 Chadwick Street, Sewickley, PA 15143
Phone: (412) 741-2810
COPYRIGHT 2005 Journal of Drugs in Dermatology, Inc.
COPYRIGHT 2005 Gale Group