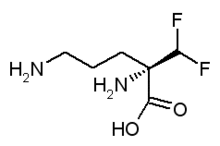
Eflornithine
Eflornithine (α-difluoromethylornithine or DFMO) is a drug manufactured by Sanofi-Aventis which has various uses. It was initially developed as a cancer medication; and while it has no significant effects on cancerous malignancies, it was found to be very effective in combatting African trypanosomiasis (sleeping sickness), in particular the West African form1 of the disease. It is hoped that eflornithine will replace the relatively toxic melarsoprol. more...
Supplies of Eflornithine are limited as it is not very cost effective to manufacture. Aventis stopped making eflornithine in 1995 because of this very reason. The drug company gave the rights to manufacture the drug to the World Health Organization, who attempted to find a new supplier. Eflornithine is also an effective hair removal agent and is the active ingredient in Vaniqa brand hair removal cream.
Eflornithine appears to work by inhibiting ornithine decarboxylase (ODC), an enzyme that regulates cell division.
Sleeping Sickness Treatment
In 2001, Aventis made a 5-year agreement with the WHO to manufacture eflornithine, melarsoprol and pentamidine, in sufficient amounts to cover existing needs. The yearly value of the drugs donated by Aventis under this agreement is US$5 million. Medecins Sans Frontieres, or Doctors Without Borders, the non-profit international medical group, assisted in the creation of the new agreement. MSF will work to distribute the drugs. In addition, under the agreement, Bristol-Myers Squibb, the manufacturer of Vaniqua, will pay for part of the eflornithine. The 5-year agreement will expire in 2006. In 2004, Aventis merged with Sanofi-Sythélabo to form Sanofi-Aventis.
The trade name of eflornithine as manufactured for the treatment of sleeping sickness is Ornidyl®.
As of September 2005, the World Health Organization's eflornithine page is reporting that the India Institute of Chemical Technology in Hyderabad, India and ILEX Oncology in Texas, United States are both working on new ways of making eflornthine more cheaply. The WHO goes on to say that ILEX is experimenting with an oral formulation of the drug as a treatment for cancer and that trials of the new oral formulation for efficacy against sleeping sickness are underway.
Hair Removal Cream
As a topical application, the drug has been shown to be an effective hair growth retardant in some patients, and is sold under the brand name Vaniqa® (eflornithine hydrochloride 13.9%). Efficacy data submitted to FDA observed about 58% of women using it on facial hair had improvement2. This study suggested it may be particularly effective in postmenopausal women. One large published study on safety found the product rarely caused significant side effects such as acne, follicle irritation, itching or dryness3. This corroborates unpublished data submitted to FDA showing about 2% of subjects discontinued use due to adverse reactions.
Read more at Wikipedia.org