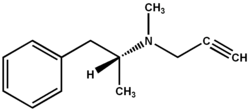
L-deprenyl (Selegiline) used in the treatment of Parkinson's and Alzheimer's disease also enhances longevity. Oxidized low density lipoprotein promotes atherosclerosis and is toxic to both vascular and neural tissue. The reported association between vascular dysfunction and neurodegenerative diseases prompted us to investigate the effect of l-deprenyl, a MAO-B inhibitor, on low density lipoprotein (LDL) oxidation. LDL was isolated from freshly collected blood and the kinetics of copper induced oxidation of LDL was monitored continuously by spectrophotometry. Oral administration (10 mg) or in vitro (2.8 to 84 (mu)M) addition of l-deprenyl inhibited oxidation of LDL isolated from healthy men and post-menopausal women. This is the first report demonstrating that the antioxidant action of l-deprenyl may be anti-- atherogenic and cardioprotective. Such an action could contribute to reported extension of life span associated with long-term administration of the drug. In conjunction with inhibition of LDL oxidation, l-deprenyl is unique in that it demonstrates protective effects on both vascular and neuronal tissue. Prophylactic use of low doses of l-deprenyl may accord protection against vascular and neurodegenerative diseases associated with aging. [Neurol Res 2002; 24: 169-173]
Keywords: L-deprenyl; LDL oxidation; Alzheimer's disease; atherosclerosis; Parkinson's disease
INTRODUCTION
The major cholesterol-carrying lipoprotein in the serum is low density lipoprotein (LDL) and high levels of the lipoprotein are associated with an increased risk for atherosclerosis1,2. Evidence also implicates free radical generation, lipid peroxidation and oxidative modification of LDL in the initiation and accelerated progression of atherosclerosis and coronary heart disease (CHD)3,4. The following lines of evidence suggest a prominent role for LDL in the atherogenic process: oxidative modification of LDL occurs in vivo5, presence of epitopes of oxidized LDL and antibodies in plasma and atherosclerotic lesions of experimental animals and humans6-9, increased susceptibility of LDL to oxidation in patients with coronary heart disease10, and decreased susceptibility of LDL to oxidation following supplementation with antioxidants such as vitamin E11. Considerable data indicates that oxidized LDL has the potential to cause cellular damage and play a pathogenic role in several clinically significant vascular and neurodegenerative diseases12. Oxidized LDL is a chemoattractant for monocytes, it is cytotoxic, and it becomes ligand for scavenger receptors (acetyl-LDL; SR)3,13. LDL is transported to the brain by a carrier and it is susceptible to oxidation in the highly oxygen- and lipid-enriched environment of the brain14. Oxidized LDL has been shown to be neurotoxic and may have a role in neurodegenerative diseases associated with aging15. Elevated LDL level has also been implicated as an independent risk factor for the development of dementia following stroke in elderly patients16. At least part of the reduction in cardiovascular morbidity and mortality associated with post-menopausal estrogen replacement therapy and antioxidants such as vitamin E and flavinoids many be mediated by amelioration of oxidized LDL toxicity17,18.
L-deprenyl (Selegiline, Eldepryl) is a monoamine oxidase-B inhibitor effective in treating Parkinson's disease (PD) and possibly Alzheimer's disease (AD)19,20. L-deprenyl is one of the few compounds that are capable of delaying functional deterioration in neurodegenerative diseases with concomitant extension of life span 21 and enhanced sexual activity22. The neuroprotective property of l-deprenyl is thought to be due to actions unrelated to the inhibition of monoamine oxidase. Recently, we reported unique vascular actions of the drug, stimulation of nitric oxide (NO) production and rapid cerebral and peripheral vasodilation by endothelium-dependent mechanisms23,24. The coexistence of depression and cardiovascular disease was first reported in 1937(25) and has recently attracted much attention. Patients with depression develop ischemic heart disease at a higher rate than nondepressed group of patients and treatment of depression reduces the associated increase in cardiovascular mortality, possibly by preventing endothelial dysfunction26,27. LDL has been shown to contribute to an inflammatory cascade by promoting leukocyte extravasation28 in CHD and Alzheimer's disease29. Interventions aimed at decreasing the susceptibility of LDL to oxidation are gathering considerable momentum along with the current emphasis on reducing serum lipids. These observations and the possibility that the increased longevity of subjects on l-deprenyl may include protective effects on the cardiovascular system prompted us to examine the effect of this neuroprotective drug on human LDL oxidation in vitro and in vivo.
METHODS
LDL isolation
Fresh whole blood was drawn by venipuncture from healthy human volunteers into vacutainer tubes containing EDTA (1 mg ml^sup -1^). The plasma was stabilized by addition of 60% (w/v) sucrose solution and used immediately for preparation of LDL or stored at -80 deg C for up to four weeks30. LDL (d =1.019-1.063 g ml^sup -1^) was prepared by single step ultracentrifugation essentially as described previously31. Before the oxidation experiments, EDTA was removed from LDL by gel filtration on Sephadex PD 10 columns and the LDL obtained was used immediately. The concentration of LDL protein was measured by the Lowry method32.
Measurement of LDL oxidation
The kinetics of oxidation of LDL was measured by continuously monitoring the change of the absorbency of the conjugated dienes at 234 nm spectrophotometrically as previously described33. The kinetics of oxidation were followed by measuring the increase in the levels of conjugated dienes at 32 deg C for 4-6 h, in the presence of 1.67 (mu)M Cu^sup 2+^. The lag phase was calculated from a plot of the change of absorbance vs. time as described30,33. In some experiments, the extent of LDL oxidation was also quantified as the formation of thiobarbituric acid reactive substances (TBARS) by previously described methods34.
Subjects
Six healthy post-menopausal women and two healthy adult men were recruited to participate in the study. All subjects were nonsmokers and were not taking any hormones or antioxidant supplements. This study was approved by the Human Research Ethics Board of the University and Hospital.
RESULTS
In vitro effect of 1-deprenyl on oxidation of low density lipoprotein
Determination of the oxidation resistance (lag-phase) of LDL by continuous monitoring of the formation of conjugated dienes
Low density lipoprotein (LDL) isolated from healthy, normolipidemic, male donors after an overnight fast was incubated with increasing concentrations (2-85 (mu)M) of l-- deprenyl and the mixture subjected to Cu^sup 2+^-mediated oxidation. The time course (Figure 1A,B) shows three phases: a lag or inductive phase during which the diene absorption changes marginally, a propagation phase during which the diene absorption increases rapidly, and finally a decomposition phase. The data shown in Figure IA indicate a dose-dependent inhibition of LDL oxidation. At 8.4 (mu)M l^sup -1^ l-deprenyl protected LDL from Cu^sup 2+^-mediated oxidation with an increase in lag phase of 10% over that observed with untreated LDL (control lag time of 56 min). At concentration of 42 and 84 (mu)M l^sup -1^ the corresponding increases in the lag phases were 57% and 93% respectively. A similar dose response was also observed with LDL isolated from a post-menopausal woman as shown in Figure 18. Similarly, LDL isolated from other normal healthy postmenopausal women (n=6) when subjected to Cu^sup 2+^-- mediated oxidation in the presence of 42 (mu)M of l-- deprenyl, resulted in an increase in the lag phase of 36% +/- 5% over that observed with untreated LDL (Figure 2). The baseline (control) lag time observed in post-menopausal women (70-78 min) was higher than that observed in man (55 min) (Figures 1 and 2). These results indicate that endogenous LDL in post-menopausal women may be better protected against oxidation than in men.
Determination of TBARS as an indicator of LDL oxidation
TBARS production was 54 +/- 5.5 nmoles mg^sup -1^ protein in LDL samples isolated from six normal healthy postmenopausal women (Figure 3). In the presence of 42 nm l^sup -1^ and 84 nm l^sup -1^ of I-deprenyl, the TBARS production decreased to 18 +/- 2 and 13.5 +/- 1.4 nmoles mg^sup -1^ protein (Figure 3), respectively. This represents a decrease of 67% and 77% in the formation of TBARS and is significantly (p
Treatment of plasma with l-deprenyl before LDL isolation
In vitro incubation of plasma from a male subject for 18 h at 37 deg C with 1.4 mMoles I^sup -1^ of l-deprenyl before the isolation of LDL also resulted in the protection of LDL against Cu^sup 2+^-mediated oxidation. The lag time increased (Figure 4) by 33% over control LDL isolated from plasma incubated for 18 h in the absence of l-- deprenyl. The results from these experiments indicate that l-deprenyl has antioxidant properties and moreover, it appears to be incorporated into the LDL particle.
In vivo effect of l-deprenyl on oxidation of low density lipoproteins
Effect of oral administration of l-deprenyl in LDL oxidation
Following ingestion of a single oral dose of 10 mg of l-deprenyl by a healthy adult male, blood samples were drawn at 0, 30, 60, 90 and 150 min. LDL was isolated and the rate of LDL oxidation was measured. The rate of diene formation and the increase in lag phase are shown in Figure 5. The lag time increased from a baseline value of 66 min to 90 min in 2.5 h following the ingestion of l-deprenyl. This clearly demonstrates that in vivo l-deprenyl has the capacity to protect LDL from oxidative modification.
DISCUSSION
In the present study, we have demonstrated that pharmacological doses of the neuroprotective drug l-- deprenyl inhibited the Cu^sup 2+^-mediated oxidation of LDL isolated from healthy adult men and women as well as post-menopausal women. The LDL oxidation was induced by Cu^sup 2+^ as this method has been shown to produce oxidized LDL that appears to have undergone the same physical changes and displays most of the properties of LDL oxidized in the presence of cells35. The baseline (control) lag time of oxidation in men was 55-66 min (Figures 1, 4 and 5) and appears to be lower than that observed in women (77-78 min, Figures I and 2). These observations suggest that LDL in women may be more protected against oxidation than in men. This is also reflected in the effect of 42 (mu)M l-deprenyl which in women prolonged the lag phase to a greater extent than in men (Figures 1 and 2). Since the number of subjects studied are few, firm conclusions regarding a potential gender difference in the effect of l-deprenyl cannot be made at the present time. Both in vitro (Figures I and 2) and in vivo (Figure 5) data clearly indicate that administration of l-deprenyl led to a prolongation of the lag-phase and a reduction of maximum rate of formation of conjugated dienes. To our knowledge this is the first report that the antioxidant action of l-deprenyl may be antiatherogenic and cardioprotective. Whether this novel antioxidant property of l-deprenyl is independent of its established role as an inhibitor of MAO-B activity remains to be investigated. The action of other MAO inhibitors on LDL oxidation is currently being investigated.
The precise mechanisms) by which l-deprenyl inhibits LDL oxidation is not evident from the present studies, but there are a number of possibilities. Since coincubation of l-deprenyl and plasma also led to protection of LDL against oxidative damage, it would appear that l-deprenyl gets incorporated into the LDL particle in a manner similar to the incorporation of vitamin E into LDL. This incorporation of l-deprenyl may change the LDL surface properties such that it is protected from oxidation. Alternatively, l-deprenyl may function as a chain-breaking antioxidant with the ability to act as a scavenger of reactive oxygen species produced by various cells including arterial wall and macrophages. In addition, l-deprenyl may also act by directly sequestering endogenous metal ions such as copper and iron. L-deprenyl may also function synergistically with other antioxidants such as vitamin E and beta-carotene associated with the LDL particle. Moreover, like vitamin C, l-deprenyl may also diminish the prooxidant action of vitamin E on LDL oxidation 36. The data presented in the present study (Figures 1 and 2) indicates that after the initial lag phase, the oxidation of LDL proceeds quite rapidly (propagation phase), suggesting that once l-deprenyl is exhausted, rapid oxidation of LDL resumes. Even though a single oral dose of l-- deprenyl showed a protective action against LDL oxidation (Figure 5), long-term administration of l-- deprenyl may be required to maintain a prolonged in vivo antioxidant effect.
In the current report, we demonstrate that in vitro the neuroprotective drug l-deprenyl protected human LDL from oxidative modification. NO and NO donors also prevent endothelial cell-mediated oxidation of LDL37. Recently, we demonstrated that l-deprenyl has a protective effect on the vascular endothelium and also stimulated the production of nitric oxide23,24. Thus, l-- deprenyl is unique in that it may accord cardiovascular benefits by direct protection of LDL from oxidation and enhancement of vascular function through NO-dependent mechanisms. In view of the recent evidence showing an association between depression and cardiovascular disease23-25, it is intriguing that l-deprenyl belonging to a class of antidepressants (MAO inhibitors) shows protective effects on the vascular system. In conjunction with a protective effect on LDL oxidation, l-- deprenyl is unique in that it exhibits beneficial effects on both vascular and neuronal tissue and such actions may contribute to the enhanced longevity of individuals using MAO-B inhibitors.
REFERENCES
1 Goldstein JL, Brown MS. The low density lipoprotein pathway and its relation to atherosclerosis. Ann Rev Biochem 1997; 46: 897-930
2 Steinberg D. Lipoproteins and atherosclerosis: A look back and a look ahead. Atherosclerosis 1983; 3: 283-301
3 Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: Modifications of low-density lipoprotein that increase its atherogenecity. N Eng J Med 1989; 230: 915-924
4 Holvoet P, Collen D. Oxidation of low density lipoproteins in the pathogenesis of atherosclerosis. Atherosclerosis 1998; 137 (Suppl.): S33-S38
5 Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative-modification in vivo. Proc Natl Acad Sci USA 1989; 86: 1372-1376
6 Palinski W, Horkko S, Miller E, et at. Cloning of monoclonal antibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice - demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98: 800-814
7 Yla-Herttuala S, Palinski W, Rosenfeld ME, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbits and man. J Clin Invest 1989; 84: 1086-1095
8 Boyd HC, Gown AM, Wolfbauer G, Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. Am J Pathol 1989; 135: 815-825
9 Palinswki WS, Yla-Herttuala S, Rosenfeld ME, Butler SW, Socher SS, Parthasarathy S, Curtiss LK, Witztum JL. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Atherosclerosis 1990; 10: 325-335
10 Rijke YB, de Verwey HF, Vojelezang CJM, et al. Enhanced susceptibility of low density lipoproteins to oxidation in coronary bypass patients with progression of atherosclerosis. Clin Chim Acta 1995; 243: 137-149
11 Princen HMG, VanDuyvenvoorde W, Buytenhek R, et al. Supplementation with low doses of vitamin E protects low density lipoproteins from lipid peroxidation in men and women. Arterioscler Thrombo Vasc Biol 1995; 15: 325-333
12 Heller FR, Descamps 0, Hondekijn JC. LDL oxidation: Therapeutic perspectives. Atherosclerosis 1998; 137 (Suppl.): S25-S31
13 Sparrow CP, Parthasarathy S, Steinberg D. A macrophage receptor that recognizes oxidized lipoproteins, but not acetylated low density lipoproteins. J Biol Chem 1989; 264: 2599-2604
14 Dehouck B, Fenart L, Dehouck MP, Pierce A, Torpier G. A new function for LDL receptor: Transcytosis of LDL across the bloodbrain barrier. J Cell Biol 1997; 138: 877-889
15 Sugawa M, Ikeda S, Kushima Y, Takshima Y, Cynshi O. Oxidized LDL caused CNS neuron cell death. Brain Res 1997; 761: 165-172
16 Moroney JT, Tang M, Berglund L, Small S, et al. Low-density lipoprotein cholesterol and the risk of dementia with stroke. JAMA 1999; 282:254-260
17 Vyas S, Gangar K. Postmenopausal estrogens and arteries. Br J Obstet Gynaecol 1995; 192: 942-946
18 Aviram M, Fuhrman B. Polyphenolic flavinoids inhibit macrophage-mediated oxidation of LDL and attenuate atherogenesis. Atheroscierosis 1998; 137 (Suppl.): S45-S50
19 The Parkinson Study Group. Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. N Eng Med 1993; 328: 175-183
20 Sano M, Ernesto C, Thomas RG, et al. A controlled trial of Selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. N Eng J Med 1997; 336: 1216-1221
21 Birkmayer WJ, Knoll P, Riederer P. Increasing life expectancy resulting from adidtion of L-deprenyl to Madopar treatment in Parkinson's disease: A long-term study. J Neurol Transm 1985; 64: 113-127
22 Knoll J, Dallo J, Yan TT. Striatal dopamine, sexual activity and life
span. Longevity of rats treated with L-deprenyl. Mech Ageing Dev 1989; 46: 525-532
23 Thomas T, McLendon C, Thomas G. L-deprenyl, nitric oxide production and dilation of cerebral blood vessels. NeuroReport 1998; 9: 2595-2600
24 Thomas T. Monoamine oxidase-B inhibitors in the treatment of Alzheimer's disease. Neurobiol Aging 2000; 21: 343-348
25 Malsberg B. Mortality among patients with involution melancholia. Am J Psychiatry 1937; 93: 1231-1238
26 Rosanski A, Blumenthal AJ, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999; 99: 2192-2217
27 Roose SP, Devanand D, Suthers K. Depression: Treating the patient with comorbid cardiac disease. Geriatrics 1999; 54: 20-35
28 Ross R. Atherosclerosis - An inflammatory disease. N Eng J Med 1999; 340: 115-121
29 Thomas T, Sutton ET, Bryant MW, Rhodin JAG. In vivo vascular damage, leukocyte activation and inflammatory response induced by fi-amyloid. J Submicrosc Cytol Pathol 1997; 29: 293-304
30 Kleinveld HA, Hak-Lemmers HLM, Stalenhoef AFH, Demacker PNM. Improved measurement of low-density lipoprotein susceptibility to copper-induced oxidation. Application of a short procedure for isolating low density lipoprotein. Clin Chem 1992; 38:2066-2072
31 Chung BH, Segrest JP, Ray MJ, Brunzell JD Hokanson JE, Krauss RM, Beaudrie K, Cone JT. Single vertical spin density gradient ultracentrifugation. Meth Enzym 1986; 128: 181-209
32 Lowry OH, Rosebrough NJ, Farr AL, Randall AJ. Protein measurement with folin phenol reagent. J Biol Chem 1951; 193: 265-275
33 Esterbauer H, Striegl G, Puhl H, Rotheneder M. Continuous monitoring of in vitro oxidation of human low-density lipoprotein. Free Radic Res Commun 1989; 6: 67-75
34 Beuge A, Aust SD. Microsomal lipid peroxidation. Meth Enzym 1981; 52: 302-310
35 Parthasarathy S, Fong LG, Quinn MT, Steinberg D. Oxidative modification of LDL: Comparison between cell-mediated and copper-mediated modification. Eur Heart 1990; 11: 83-87
36 Noguchi N, Niki E. Dynamics of vitamin E action against LDL oxidation. Free Rad Res 1998; 28: 561-572
37 Hogg N, Kalyanaraman B, Joseph J, Struck A, Parthasarathy S. Inhibition of LDL oxidation by nitric oxide. FEBS 1993; 334: 170-174
Tom Thomas, Bhagu R. Bhavnani* and Prem Thomas
Woodlands Medical Center and Endron Therapeutics, Oldsmar, FL, USA
*Department of Obstetrics and Gynecology, University of Toronto, St. Michael's Hospital, Toronto, Ontario, Canada
Correspondence and reprint requests to: Dr Tom Thomas, Woodlands Medical Center, 3150 Tampa Road, Oldsmar, Florida 34677, USA. [ayurveda.treatment@verizon.net] Accepted for publication October 2001.
Copyright Forefront Publishing Group Mar 2002
Provided by ProQuest Information and Learning Company. All rights Reserved