The first oral medication to relieve the pain and discomfort of interstitial cystitis (IC), an inflammatory disease of the bladder wall, has been approved by FDA.
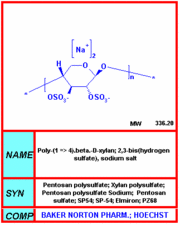
The agency approved the drug, Elmiron (pentosan polysulfate sodium), last Sept. 26 under its orphan products program, which encourages development of treatments for rare diseases.
About 450,000 people in the United States are believed to have IC. But true numbers are hard to come by, because many cases are either undiagnosed or misdiagnosed. While most cases are in women, at least 10 percent of cases are in men.
No one knows what causes IC. Although bacteria, fungi or viruses are not found in patients' urine, many researchers believe the cause may be an infectious agent that hasn't yet been identified. Another theory holds that the inner lining that protects the bladder wall from toxic effects of urine may be "leaky," allowing substances in the urine to penetrate the wall and trigger IC symptoms.
In clinical studies, at least 38 percent of IC patients responded to treatment with the new drug. How Elmiron relieves the bladder pain associated with IC is not known, but the hypothesis is that the drug improves the bladder's defective lining.
Some patients see results in weeks. A few patients may take as long as six months before getting relief. If pain is not relieved in six months, the benefits of continuing treatment are unknown.
Elmiron is a weak blood thinner; therefore, patients should tell their doctors if they are taking other drugs with a similar effect, such as anticoagulants, or high doses of aspirin or other anti-inflammatory drugs, such as ibuprofen. A package insert for patients includes information on side effects, how to take the drug, and who should take it.
IC symptoms are similar to those of a urinary tract infection. Most people have some of the following symptoms:
* urgent need for frequent urination both day and night
* reduced bladder capacity
* feelings of pressure, pain and tenderness around the bladder, pelvic and genital area, which may increase as the bladder fills and decrease as it empties
* painful sexual intercourse
* in men, discomfort or pain in the prostatic area.
Elmiron is marketed by Baker Norton Pharmaceuticals Inc., Miami, Fla. At FDA's request, the firm will conduct postmarketing studies.
(For more information, see "Interstitial Cystitis: Progress Against Disabling Bladder Condition," in the November 1995 FDA Consumer.)
COPYRIGHT 1997 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group