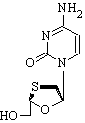
Epivir
Lamivudine (2',3'-dideoxy-3'-thiacytidine, 3TC) has the trade name Epivir®. It is a potent reverse transcriptase inhibitor of the class nucleoside analog reverse transcriptase inhibitor (NARTI). more...
Lamivudine has been used for treatment of chronic hepatitis B at a lower dose than for treatment of HIV. It improves the seroconversion of e-antigen positive hepatitis B and also improves histology staging of the liver. Long term use of lamivudine unfortunately leads to emergence of a resistant hepatitis B virus (YMDD) mutant. Despite this, lamivudine is still used widely as it is well tolerated.
History
Lamivudine was invented by Bernard Belleau and Nghe Nguyen-Ba at the Montreal-based IAF BioChem International, Inc. laboratories in 1989. The drug was later licensed to the British pharmaceutical company Glaxo for a 14 percent royalty.
Lamivudine was approved by the Food and Drug Administration (FDA) on Nov 17, 1995 for use with Zidovudine (AZT) and again in 2002 as a once-a-day dosed medication. The fifth antiretroviral drug on the market, it was the last NRTI for three years while the approval process switched to protease inhibitors. Its patent will expire in the United States on 2016-05-18.
Mechanism of action
Lamivudine is an analogue of cytidine. It can inhibit both types (1 and 2) of HIV reverse transcriptase and also the reverse transcriptase of hepatitis B. It needs to be phosphorylated to its triphosphate form before it is active. 3TC-triphosphate also inhibits cellular DNA polymerase.
Lamivudine is administered orally, and it is rapidly absorbed with a bio-availability of over 80%. Some research suggests that lamivudine can cross the blood-brain barrier. Lamivudine is often given in combination with zidovudine, with which it is highly synergistic. Lamivudine treatment has been shown to restore zidovudine sensitivity of previously resistant HIV. Several mutagenicity tests show that lamivudine should not show mutagenic activity in therapeutical doses.
Read more at Wikipedia.org