Transfer of Protoporphyrin IX Between Cells
ABSTRACT
Human adenocarcinoma cells of the line WiDr and human leukemia T cells of the line Jurkat were incubated with 5aminolevulinic acid and found to produce protoporphyrin IX (PpIX). They were able to transfer a fraction of the sensitizer to neighboring control cells. The transfer took place through direct membrane contact. Light exposures, inactivating about 20% of the sensitized cells, did not result in any acceleration of the transfer of PpIX. This is in contrast to what has been reported for PpIX in erythrocytes from patients with erythropoietic protoporphyria. In these cells light exposure transfers PpIX from the binding sites on hemoglobin to the plasma membrane and further to neighboring cells. The lack of light-induced transfer in the WiDr and Jurkat cells may be related to the binding sites of PpIX, supposedly membrane lipids and proteins embedded therein. Light exposure slightly increased the rate of loss of PpIX from WiDr cells.
Abbreviations: ALA, 5-aminolevulinic acid; EPP, erythropoietic protoporphyria; FCS fetal calf serum; PDT, photodynamic therapy; PpIX, protoporphyrin IX.
INTRODUCTION
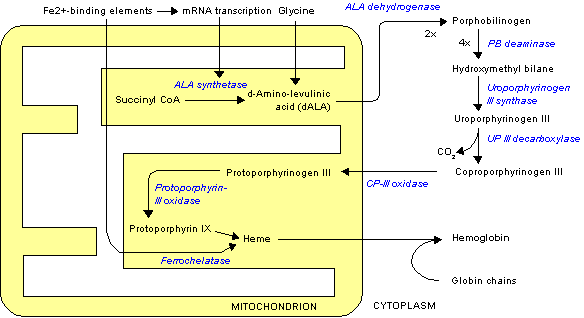
When added to cells, protoporphyrin IX (PpIX) and other lipophilic sensitizers are usually bound to membrane lipids and proteins embedded in these lipids (1). The same is true for PpIX generated intracellularly from exogenously added 5-aminolevulinic acid (ALA) (2). We have shown earlier that light exposure may relocalize sensitizers in cells. This can be seen either by means of fluorescence microscopy or, as in the case of porphyrins, by a change in the quantum yield of photoinactivation of the cells occurring during light exposure (3-5). Light exposure results not only in relocalization of PpIX within cells (6) but also in release of PpIX from intact cells (7) and in transfer of PpIX between cells (8). When erythropoietic protoporphyria (EPP) red cells were layered on top of a monolayer of tissue-cultured cells and exposed to light, PpIX was detached from hemoglobin inside the EPP cells and transferred to their plasma membrane and further to neighboring cells (8). This probably explains why the PpIX concentration in erythrocytes of EPP patients is lower in the summer season than in the winter season (9) and the observation that these patients experience a much higher skin photosensitivity on the day after a day of mild light-induced cutaneous symptoms (10). Thus, the first light exposure may have transferred some PpIX from EPP erythrocytes to endothelial cells in the skin. The second exposure, then, leads to severe skin damage related to the PpIX that was transferred from the EPP cells in the blood to the skin by the first exposure.
ALA-photodynamic therapy (ALA-PDT) is a modem version of treatment of actinic keratosis and some forms of skin cancer. ALA is administered in a cream, and PpIX is produced with some selectivity in the skin lesion. Light exposure leads to inactivation of the malignant cells. This form of therapy closely resembles the skin sensitization experienced by EPP patients. In the present work we have investigated whether ALA-induced PpIX can be transferred between cells and whether light exposure has any influence on such transfer. Two cell lines were investigated: one from a solid tumor (WiDr) and one line of suspension cells (human leukemia T cells).
MATERIALS AND METHODS
Chemicals. 5-Aminolevulinic acid, provided by Sigma Chemical Co. (St. Louis, MO), was dissolved in Dulbecco's phosphate-buffered saline, and the pH value was adjusted to 7.0 by addition of 5 M NaOH. The solutions were diluted in serum-free RPMI-1640 medium.
Cell cultivation. WiDr cells, derived from a primary adenocarcinoma of the rectosigmoid colon, were subcultured in RPMI-1640 medium (GIBCO, Grand Island, NY) containing 10% fetal calf serum (FCS). The cells were seeded in 10 cm2 tissue culture wells and grown for 3 days before ALA was added. The cell density then corresponded to an almost confluent monolayer. Some experiments were carried out with suspension cells of the human leukemic T cell line Jurkat. These cells are derived from an acute lymphoblastic leukemia and kept in exponential growth in RPMI-1640 medium with 10% FCS.
ALA incubation. The WiDr cells were incubated with 1 mM ALA in serum-free medium for 2 h. Then they were trypsinized, washed, resuspended in serum-free medium and allowed to sediment on a monolayer of WiDr cell that had not been in contact with ALA. In some experiments the trypsin solution was removed, whereas in other experiments the trypsin solution was present during the period of contact between the two cell populations. After different time periods all cells were brought into suspension by trypsinization.
The Jurkat cells were incubated for 4 h with serum-free medium containing 1 mM ALA. Then they were washed and mixed with Jurkat cells that had not been incubated with ALA. Both cell populations were allowed to sediment and stay together for different time periods before analysis.
Light exposure. In some experiments the mixed cell populations (cells incubated with and without ALA) were exposed for 5 min to the light from a bank of fluorescent light tubes (Model 3026, Applied Photophysics, London, UK) with an irradiance of 2.3 mW/cm^sup 2^. The emission was mainly in the wavelength region 370-450 nm, with a peak at 405 nm, which is close to the maximum of the Soret band of PpIX in cells.
Cell survival. Survival curves of the WiDr cell were determined by the usual method of colony formation. The numbers of surviving and colony-forming cells were scored after 11 days incubation. The survival level of Jurkat cells was estimated by microscopically determining the number of intact cells after 2 h.
Analysis by flow cytometry. The PpIX fluorescence of the cells was measured by means of flow cytometry. A Becton Dickinson (San Jose, CA) instrument was used. The excitation wavelength was 488 nm (i.e. within the fluorescence excitation spectrum of PpIX). At the detection side a 620-640 band-pass filter was placed in front of the photomultiplier. The light-scattering signal from the cells was also registered.
RESULTS
As shown in Figs. 1 and 2, there is a transfer of PpIX from sensitized to unsensitized WiDr cells. This transfer is mediated by cell contact because addition of medium from sensitized cells to unsensitized cells did not result in any PpIX fluorescence in the latter cells. There was no significant PpIX fluorescence detected in the medium either. The transfer was almost complete within 30-50 min. Surprisingly, light exposure did not result in any acceleration of the transfer of PpIX between the cells (Fig. 2b). However, lightexposed sensitized cells lost their PpIX somewhat faster than the unexposed cells did (Fig. 2). Because this increased loss did not result in any increased uptake of PpIX in the unsensitized cells, we may assume that some of the lost PpIX remained in the medium, although the fluorescence of the medium was too weak to make determinations of PpIX concentrations possible. Because the medium did not exhibit any significant fluorescence, it seems that the PpIX may be aggregated. It is well known that PpIX, being lipophilic, aggregates in aqueous solutions.
The presence of trypsin during the period of cell contact led to a slightly slower loss rather than to a faster one of PpIX from sensitized cells (Fig. 3). This was true irrespective of light exposure (Fig. 2a,b). However, the presence of trypsin seemed to increase the rate of uptake of PpIX into unsensitized cells (Figs. 2 and 3).
The light exposure (5 min) was chosen to be on the shoulder of the survival curve, as shown in Fig. 4. About 80% of the cells survive a 5 min exposure. This exposure does not result in any marked morphological changes of the cells. Microscopic examination of the suspended Jurkat cells indicated that their survival level after a 5 min light exposure was similar to that of the WiDr cells.
In the Jurkat cells unexposed to light, the PpIX synthesis continued after removal of the ALA (Fig. 5). In light-exposed cells no such synthesis took place, and the PpIX concentration decreased slightly (Fig. 5b). Irrespective of light exposure, some PpIX was transferred to unexposed cells (Fig. 5). All experiments were reproduced at least two times, with consistent results.
DISCUSSION
The present work clearly shows that PpIX is transferred between cells in contact with each other. This is true for cells in monolayers as well as for cells in suspension. The transfer goes primarily via direct membrane-to-membrane contact. This is in agreement with the fact that PpIX is a lipophilic compound that tends to localize in membrane lipids, partly bound to proteins embedded therein (2). It is likely that membrane contact facilitates PpIX transfer. It should be noted that only a small fraction of the PpIX lost from the loaded cells is taken up by unloaded cells. We propose that the rest of the PpIX lost from the loaded cells aggregates and gets nonfluorescent in the aqueous medium. Alternatively, it might bind to the surface of the tissue-culture vessel.
Surprisingly, light exposure did not accelerate the intercellular transfer of PpIX, although it resulted in a slightly increased rate of loss of PpIX from loaded cells in the absence of trypsin (Fig. 2b). This is in contrast with what was expected. Thus, it has been reported that light exposure of erythrocytes from patients with EPP leads to transfer of the PpIX from its initial binding site on hemoglobin in the cells to the plasma membrane and further to albumin in the serum or to neighboring cells (6-8). Furthermore, we have observed that the quantum yield of photoinactivation of cells in the presence of Photofrin, which has a similar lipophilicity to PpIX, and, in fact, contains some PpIX, changes during light exposure (5). We attributed this to intracellular relocalization of the sensitizer (5). Membrane proteins appear to be crosslinked by PDT (1), and the binding sites of PpIX on such proteins are destroyed by light exposure (2). This may explain the increased rate of PpIX loss observed (Fig. 2b).
The reason for the difference between the cells studied in the present work and EPP erythrocytes, with respect to transfer of PpIX, is probably related to the fact that in the EPP cells PpIX is bound to hemoglobin and not to the plasma membrane. Therefore, the rate of loss of PpIX is low in the absence of light but strongly increased when the binding sites on hemoglobin are destroyed and PpIX is forced to move to the plasma membrane, where it gets in contact with the extracellular environment.
Trypsinization changes membrane proteins slightly but not in such a way that the pharmacokinetics of PpIX is significantly altered.
The Jurkat T cells differ from the WiDr cells in one respect: they continue to produce PpIX even after ALA is removed (Fig. 5a).
This has been observed also for other cells, but usually only after shorter incubation times with ALA (11). We conclude that after 4 h of ALA incubation the Jurkat cells have accumulated a reservoir of ALA that can be used for further PpIX synthesis. PDT seems to inhibit this synthesis (Fig. 5b). Under certain conditions ALA-PDT reduces the rate of PpIX synthesis in other cell lines and in mouse skin as well (data not shown).
The intracellular transfer of PpIX may play a minor role in the sensitization of skin tumors after topical application of ALA. Only a small fraction of the PpIX lost from loaded cells is transferred to neighboring cells. However, transfer may play an important role in split-dose treatments. The first part of the split dose will degrade most of the PpIX, and then PpIX diffusion or transfer from neighboring tissues may contribute to reappearance of PpIX in the target tissue before the second fraction of light is given.
Acknowledgements-The present work was supported by the Norwegian Cancer Society. The authors thank Vladimir lani, Elm Kristiansen and Kirsti Solberg Landsverk for excellent technical assistance.
Para Posted on the web site on 30 August 2002.
REFERENCES
1. Moan, J. and A. I. Vistnes (1986) Porphyrin photosensitization of proteins in cell membranes as studied by spin-labelling and by quantification of DTNB-reactive SH-groups. Photochem. PhotobioL 44 15--19.
2, Moan, J., G. Streckyte, S. Bagdonas, 0. Bech and K. Berg (1997) Photobleaching of protoporphyrin IX in cells incubated with 5aminolevulinic acid. Int. J. Cancer 70, 90-97.
3, Moan, J., K. Berg, E. Kvam, A. Western, Z. Malik and A. Ruck (1989) Intracellular localization of photosensitizers. In Photosensitizing Compounds: Their Chemistry, Biology and Clinical Use, pp. 95-111. Wiley, Chichester, Ciba Foundation Symposium 196.
4, Moan, J., H. Anholt and Q. Peng (1990) A transient reduction of the fluorescence of aluminium phthalocyanine tetrasutphonate in rumours
during photodynamic therapy. J. Photochem. Photobiol. B Biol. 5, 115-119.
5. Moan, J. (1988) A change in the quantum yield of photoinactivation of cells observed during photodynamic treatment. Lasers Med. Sci. 3, 93-97.
6. Brun, A. and S. Sandberg (1988) Light-induced redistribution and photobleaching of protoporphyrin in erythrocytes from patients with erythropoietic protoporphyria: an explanation of the rapid fading of fluorocytes. J. Photochem. Photobiol. B Biol. 2, 33-41.
7. Brun, A. and S. Sandberg (1985) Photodynamic release of protoporphyrin from intact erythrocytes in erythropoietic protoporphyria: the effect of small repetitive light doses. Photochem. Photobiol. 41, 535 541.
8. Brun, A., A. Western, Z. Malik and S. Sandberg (1990) Erythropoietic protoporphyria: photodynamic transfer of protoporphyrin from intact erythrocytes to other cells. Photochem. Photobiol. 51, 573-577.
9. Lin Miao, L., M. M. Mathews-Roth and M. B. Poh-Fitzpatrick (1979) Beta carotene treatment and erythrocytic protoporphyrin levels. Arch. Dermatol. 115, 818.
10. Poh-Fitzpatrick, M. B. (1989) The "priming phenomenon" in the acute phototoxicity of erythropoietic protoporphyria. J. Am. Acad. Dermatol. 21, 311.
11. Moan, J., K. Berg, 0. B. Gadmar, V. lani, L. W. Ma and P. Juzenas (1999) The temperature dependence of protoporphyrin IX production in cells and tissues. Photochem. Photobiol. 70, 669-673.
Johan Moan, Li Wei Ma* and Trond Stokke
Department of Biophysics, The Norwegian Radium Hospital, Oslo, Norway
Received 7 June 2002; accepted 20 August 2002
*To whom correspondence should be addressed at: Department of Biophysics, The Norwegian Radium Hospital, 0310 Montebello, Oslo, Norway. Fax: 47-22-93-4270; e-mail: johan.moan@labmed.uio.no
Copyright American Society of Photobiology Nov 2002
Provided by ProQuest Information and Learning Company. All rights Reserved