Summary points
The porphyrias form a group of inherited disorders of haem biosynthesis of which there are seven main
Porphyrias can be classified into acute (neuropsychiatric), cutaneous, and mixed forms
Acute forms can be life threatening, but attacks can be aborted by early administration of haem arginate
The acute porphyrias are often misdiagnosed; most commonly they present as acute abdominal pain or as neurological or atypical psychiatric symptoms
Patients with porphyria should be referred to specialist centres and be advised to avoid precipitating factors, such as certain drugs
When a patient is diagnosed with an acute porphyria the whole family needs to be screened
Although porphyria is a relatively uncommon condition, it should be considered in patients presenting with an atypical medical, psychiatric, or surgical history. Acute attacks are associated with a substantial morbidity and mortality; there is a need for rapid and accurate diagnosis of the neuropsychiatric porphyrias, particularly because haem arginate can induce a definite remission if given early in an attack. Additionally, porphyrias may present with skin lesions or photosensitivity.
What are the porphyrias?
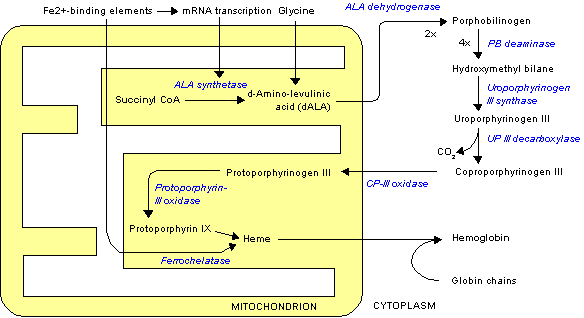
The porphyrias form a heterogeneous group of inherited disorders of haem biosynthesis, and they are often missed or wrongly diagnosed. A partial deficiency of one of the seven enzymes in the pathway causes characteristic clinical and biochemical features. These disorders are due to a specific alteration in the pattern of accumulation of porphyrin and porphyrin precursors (table). Each type of porphyria is defined by a unique pattern of accumulation and excretion of haem precursors, as well as a reduction in the relevant enzyme activity. Correct interpretation of the appropriate biochemical investigations is essential for accurately diagnosing and managing the porphyrias, as clinical features alone are not sufficiently specific either to confirm a diagnosis or to distinguish between the various forms.
Summary of diagnosis patterns of overproduction of haem precursors in different porphyrias
(*) Lead poisoning produces an identical overproduction pattern.
There are seven main types of porphyria (fig 1), which are broadly classified according to clinical features into neuropsychiatric, dermatological, and mixed forms. Acute intermittent porphyria and plumboporphyria are predominantly neuropsychiatric; congenital erythropoietic porphyria, porphyria cutanea tarda, and erythropoietic protoporphyria have predominantly cutaneous manifestations; and hereditary coproporphyria and variegate porphyria are classified as mixed as they may have both cutaneous and neuropsychiatric features. The prevalence of porphyria varies widely from country to country and also depends on the type of porphyria. Overall prevalence of overt cases in the United Kingdom is about 1 in 25 000 population for porphyria cutanea tarda and less than 1 in one million for congenital erythropoietic porphyria.[1] Plumboporphyria has not been reported in Britain.
[Figure 1 ILLUSTRATION OMITTED]
Methods
We based this article on literature reviews, including a Medline search (1966-98), and on the many years' experience of our department in the management of the porphyrias. King's College Hospital is one of the two recently established supraregional assay service centres for laboratory diagnosis and for the provision of clinical advice on the management of porphyria.
General clinical aspects
Patients with porphyria present in three different ways--with cutaneous lesions, acute attacks (see below for features), or both. Clinically identical acute attacks can occur in acute intermittent porphyria, variegate porphyria, hereditary coproporphyria, and plumboporphyria.[2] Skin lesions accompany the acute attack in about half of patients with variegate porphyria and in about a third of patients with hereditary coproporphyria[2]; skin lesions may be the sole presentation in these porphyrias.
Acute attacks
Acute intermittent porphyria is the commonest of the acute porphyrias. The clinical features of an acute attack vary greatly. The most common symptom is severe abdominal pain, which may be accompanied by neurological and psychiatric symptoms (fig 2).[3] Muscular weakness, particularly a proximal myopathy affecting the arms, is common. Muscular weakness can, however, progress to quadraparesis and respiratory paralysis and arrest, which may resemble the Guillain-Barre syndrome. Mild sensory changes often accompany the predominantly motor neuropathy--often in a "bathing trunk" distribution.[4]
[Figure 2 ILLUSTRATION OMITTED]
Marked constipation, nausea, vomiting, postural hypotension, and hypertension are other common features. Hyponatraemia occurs as a result of dehydration, nephrotoxicity, or occasionally inappropriate antidiuretic hormone secretion. The pathogenesis of the clinical features is poorly understood, but possible mechanisms include damage by free radicals,[5] direct neurotoxicity of aminolaevulinic acid,[6] and haem deficiency in nervous tissue.[7] The recent development of an animal model of gene knockout acute intermittent porphyria[8] should allow rapid progress in this area.
The acute attacks are often triggered by exposure to exogenous precipitating factors,[9 10] including a wide range of commonly prescribed drugs[11]; illicit drugs including amphetamines, cocaine, and derivatives; alcohol misuse; fasting; stress; infection; sex hormone treatment; and smoking (box).[12] In women relapses occur particularly premenstrually and in pregnancy. Acute attacks are rare before puberty, are most common in people in their 30s, and are four to five times more common in females than in males, with a peak age of presentation in the early 30s. Only 10-15% of gene carriers develop the clinical syndrome. A third of patients have no family history, the condition probably having remained latent (inactive) or unidentified for several generations. The frequency and severity of the attacks vary widely. In some people the disease remains latent throughout life, even in the presence of precipitating factors. Other people experience frequent and sometimes life threatening attacks, even in the apparent absence of exogenous precipitating factors.
Drug treatment should be prescribed only after reference to a drug list. Many such lists exist,[11 13] but no list is universally accepted. Some drugs are generally agreed to be safe in acute porphyria; some are considered to be unsafe; and a large number of drugs may or may not be safe. For this last group of drugs, a commonsense assessment of benefit versus risk is needed; an acute attack is less likely to be precipitated if the disease is latent, if the patient has previously only had a single attack, and if the concentrations of urinary porphobilinogen and particularly of aminolaevulinic acid are normal at the time of prescribing the drug.
Laboratory diagnosis
When a porphyric patient presents with symptoms of a possible acute attack, the key question is whether these are due to porphyria; not all symptoms in porphyric patients are due to porphyria--porphyric patients are not immune to other conditions.
Acute attacks of porphyria are invariably associated with raised urinary excretion of aminolaevulinic acid and porphobilinogen. A fresh urine sample, protected from light, should be sent to a specialist laboratory for accurate quantitation of aminolaevulinic acid and porphobilinogen concentrations. Routine laboratory screening tests may be unreliable,[14] but a recently introduced screening kit seems promising.[15] A clue to the diagnosis is that the urine is often dark on standing owing to polymerisation of porphobilinogen to porphyrins and other pigments. Between attacks, however, concentrations of urinary porphobilinogen and particularly aminolaevulinic acid are often normal. Plasma fluorescence is usually increased in variegate porphyria and is valuable both diagnostically and for family studies.[16 17]
Acute intermittent porphyria, variegate porphyria, and hereditary coproporphyria are differentiated by analysis of faecal porphyrins (table). The diagnosis of acute intermittent porphyria can be further confirmed by the demonstration of reduced red cell hydroxymethylbilane synthase activity,[2] although it is important to check that routine haematological indices are normal. DNA analysis is not yet of routine diagnostic value.
Management of acute attacks
About 1% of acute attacks of porphyria may be fatal. Most patients with an acute attack will require admission to hospital. Only drugs known to be safe in porphyria should be prescribed. For severe pain, opiates are safe--that is, non-porphyrogenic. Pethidine, morphine, or diamorphine can be given. Chlorpromazine may be helpful to promote relaxation and sleep. Sympathetic overactivity causing tachycardia and hypertension can be alleviated with propranolol.
Convulsions may occur during an acute attack. Their onset may be precipitated by hyponatraemia, so plasma osmolality and electrolyte values should always be checked.[2] Treatment is by fluid restriction, and the convulsions usually resolve as the attack subsides. Rarely, epilepsy may be the presenting symptom in acute intermittent porphyria. Treatment should be aimed at the underlying disease. Seizures are notoriously hard to manage in porphyria as all commonly used anticonvulsant agents are porphyrogenic and may therefore lead to further exacerbation of the disease.[18 19]
Historically, bromides have been the drug of choice for seizure control in most patients with acute porphyria.[18 20] However, in the mid to late 1990s several new antiepileptic drugs were introduced, and, of these, gabapentin[21-23] and vigabatrin[21] were shown to be successful in seizure control without inducing porphyric crises. These drugs are now the treatment of choice for these patients.
Oral and intravenous glucose (for maintaining a high energy intake) and haem arginate are the mainstay of treatment. They reduce synthesis of aminolaevulinic acid, resulting in a clinical and biochemical remission, with urinary excretion of aminolaevulinic acid and porphobilinogen falling towards normal values. Glucose (10% intravenously) should be given for mild attacks and pending administration of haem arginate.
Haem arginate (Normosang, Leiras Medica, Finland) should be given at an early stage in an attack[24] as it cannot reverse an established neuropathy. It is given at a dose of 3 mg/kg/day for 4 days over 15 minutes by slow intravenous infusion. It is highly irritant so should be given by a central venous line. It acts rapidly and has a dramatic effect within one week. It is expensive and is available on an urgent need basis from the company (Orphan Europe, Henley-on-Thames, Oxfordshire) or from the two supraregional assay service centres. Recently tin protoporphyrin, an inhibitor of haem oxygenase, has been shown to prolong remission when given with haem arginate.[25] Its side effects of cutaneous photosensitivity and potential toxicity limit its use, however, and further evaluation is continuing. A small proportion of patients--mainly females--have recurrent attacks with no apparent precipitants. Prophylactic haem arginate may be necessary on a regular basis for such patients. Induction of a chemical menopause with luteinising hormone releasing hormone agonists has been successfully used in this situation.
Prevention of attacks
Precipitating factors mentioned previously (such as alcohol, smoking, and porphyrogenic agents) should be avoided, as should sudden ox' prolonged low energy diets. Patients should wear a Medic Alert bracelet (an emergency identification bracelet) and should be fully educated regarding precipitating factors. They should be given an information booklet, be encouraged to join a support group--for example, the British Porphyria Association (14 Mollison Rise, Gravesend, Kent DA12 4QJ)--and be referred to a specialist centre. Attacks may occur during pregnancy, when oestrogen concentrations are high, and the patient should be advised to avoid pregnancy until she has been in remission for at least two years. Aminolaevulinic acid can cross the placenta and possibly cause toxicity to the developing fetal brain.[26] If acute attacks do occur during pregnancy they should be treated in the normal way. Haem arginate has been used successfully in pregnancy.
Cutaneous porphyrias
Photosensitisation is due to accumulation of porphyrins in the skin and may present as one of two distinct syndromes. Erythropoietic protoporphyria presents in childhood with acute photosensitivity--that is, burning, itching, and erythema on exposure to sunlight, but without bullae and with minimal scarring--and is due to an inherited partial deficiency of ferrochelatase.[27] The disease usually presents in childhood, and there is also accumulation of protoporphyrin in the liver leading to cholelithiasis and occasionally liver failure.
Skin lesions in the remaining cutaneous porphyrias (porphyria cutanea tarda, variegate porphyria, hereditary coproporphyria, and congenital erythropoietic porphyria) are similar and include fragile skin that heals slowly after minor trauma, subepidermal bullae (fig 3), pigmentation, and hypertrichosis, particularly on the forehead and upper cheeks. The lesions occur in skin exposed to the sun and are most severe in congenital erythropoietic porphyria.[28] Porphyria cutanea tarda is the commonest of all the porphyrias (table). Most cases are associated with liver cell damage. There are two forms.
[Figure 3 ILLUSTRATION OMITTED]
Sporadic porphyria cutanea tarda--The sporadic form accounts for 80-90% of all cases of porphyria cutanea tarda. Aetiological factors include alcohol, oestrogens, iron, and chemicals (for example, hexachlorobenzene). There is also an association with hepatitis C. Mild iron overload is nearly always present. People with genetic haemochromatosis are four times more likely than otherwise normal subjects to have sporadic porphyria cutanea tarda,[29] and these patients need to be investigated for iron overload--for example, ferritin and liver iron concentrations and DNA studies for the coexistence of genetic haemachromatosis.
Familial porphyria cutanea tarda--The familial form accounts for 10-20% of all cases of porphyria cutanea tarda. Autosomal dominant hepatoerythropoietic porphyria (a severe rare homozygous form) also exists, but environmental factors are also important in the expression of the disease. Biochemically, minor rises in liver toxicity and ferritin concentrations are common. Liver biopsy usually shows fatty infiltration and inflammatory changes, with cirrhosis being present in a third of cases.[30] Porphyria cutanea tarda increases the risk of hepatocellular carcinoma in patients with chronic liver disease.[30-32] Treatment of the contributory hepatotoxic agents--for example, abstention for alcohol misuse, venesection for iron overload, and, somewhat controversially, chloroquine therapy--can lead to a useful improvement in the skin lesions, with long term remission in some patients.[33] Chloroquine forms a complex with uroporphyrin and promotes release of uroporphyrin from the liver; it may also inhibit the synthesis of uroporphyrin.[34] Use of ultraviolet blockers is also valuable.
Laboratory diagnosis of cutaneous porphyrias
Erythropoietic protoporphyria is diagnosed or excluded by measuring erythrocyte free protoporphyrin. Analysis of urine and faeces is helpful (table).
In patients who are suspected of having one of the remaining cutaneous porphyrias it is essential to examine both urine and faeces for excess porphyrins as the water solubility (and hence route of excretion) of the individual porphyrins differs. Positive findings should be followed up by fractionation to identify and quantitate the individual porphyrins (including isomers). This will usually entail referral to a specialist laboratory with experience of the porphyrias. It is particularly important to distinguish between those patients with variegate porphyria and hereditary coproporphyria presenting with skin lesions alone (who will be at risk of from life threatening acute neurological attacks if exposed to precipitants) and those with porphyria cutanea tarda or congenital erythropoietic porphyria (who are not at risk from such attacks).
Management of cutaneous porphyrias
The cutaneous porphyrias are treated by avoidance of sunlight and attention to skin care. Additionally, venesection to deplete excess iron stores, oral chloroquine to increase urinary porphyrin excretion, and avoidance of alcohol and oestrogens are all particularly helpful in porphyria cutanea tarda. In erythropoietic protoporphyria, in addition to the above, administration of carotene[35] is often helpful and is believed to work by quenching active oxygen species.
Screening and family studies
The dominant mode of inheritance of the acute porphyrias, the occurrence of asymptomatic gene carriers, and the risk of developing potentially fatal attacks of neuropsychiatric origin if someone is exposed to a wide range of common precipitating factors make it essential to exclude or confirm the diagnosis of neuropsychiatric porphyria in all relatives whenever this has been diagnosed in any family member. In some cases, however, because of the equivocal biochemical analyses, confirming or refuting the inheritance of acute porphyria is not always possible. In these cases, genetic analysis is increasingly used, but this is sometimes difficult. In acute intermittent porphyria, for example, there are over 100 mutations including deletions, insertions, and missense, nonsense, and splicing mutations, and most of these mutations are family specific.[36] However, the gene locations of many of the porphyrias have been identified,[1 11] and rapid progress is expected in this area.
Clearly, families benefit when the mutation is detected. Not only will detection identify members with latent porphyria, but also unaffected members can be distinguished with confidence.[36] Exclusion of carrier status makes it unnecessary to follow restrictions to prevent attacks and avoids the need for screening of future generations of that particular family branch.
We thank Dr N Dani (general practitioner), Dr Z Yahya, and Ms Leah Mesirow for help in preparing the manuscript and figures. We also acknowledge the valuable comments of the anonymous referee.
Competing interests: None declared.
Factors that may precipitate acute attacks of porphyria
* Drugs--barbiturates and oestrogens (may be safe in replacement doses), progesterones, sulphonamides methyldopa, danazol, diazepam, phenytoin, carbamazepine, sulphonylureas, chloramphenicol, tetracyclines, some antihistamines
* Fasting
* Smoking
* Alcohol
* Substance misuse--particularly marijuana ecstasy, amphetamines, and cocaine
* Infection
* Emotional and physical stress
* Cyclic factors--premenstrual attacks are common
[1] Elder GH, Smith SG, Jane Smyth S. Laboratory investigation of the Porphyrias. Ann Clin Biochem 1990;27:395-412.
[2] Kappas A, Sassa S, Galbraith PA, Nardaman Y. The porphyrias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic basis of inherited diseases. 6th ed. New York: McGraw-Hill, 1989:1305-66.
[3] Thadani H, Wassif W, Deacon A, Peters T. Neuropsychiatric manifestations of acute intermittent porphyria. J Psychiatric Case Reports 1997;2: 29-35.
[4] Elder GH, Hilt RT, Meissner PN. The acute porphyrias. Lancet 1997;349:1613-7.
[5] Monterio H, Bechara EJH, Abdalla DSP. Free radicals involvement in neurological porphyrias and lead poisoning. Mol Cell Biochem 1991;103:73-84.
[6] Bonkovsky HL. Advances in understanding and treating "the little imitator", acute porphyria. Gastroenterology 1993;105:590-4.
[7] Lindberg RLP, Parcher C, Grandchamp B. Porphobilinogen deaminase deficiency in mice causes a neuropathy resembling that of human hepatic porphyria. Nat Genet 1996;12:195-9.
[8] De Verneuilh, GC, Boulechfar S, Moreau-Gaudry F. Porphyrias: animal models and prospects for cellular and gene therapy. J Bioenerg Biomed 1995;27:239-48.
[9] Meyer UA, Schuurmans MM, Lindberg RL. A review of the pathogenesis of the neurological manifestations of the acute porphyrias. Seminars in Liver Disease 1998;18:43-52.
[10] Kauppinen R, Mustajoki P. Prognosis of acute porphyria: occurrence of acute attacks, precipitating factors, and associated diseases. Medicine 1992;71:1-13.
[11] McColl KEL, Dover S, Fitzsimons E, Moore MR. Porphyrin metabolism and the porphyrias. In: Weatherall DJ, Ledingham JGG, Warrell DA, eds. Oxford textbook of medicine. Oxford: Oxford University Press, 1996: 1388-99.
[12] Lip GYH, McColl KEL, Goldberg A, Moore RA. Smoking and recurrent attacks of acute intermittent porphyria. BMJ 1991;302:507-8.
[13] Disler PB, Moore MR. Drug-sensitive diseases: Acute porphyrias. Adverse Drug Reaction Bulletin 1989; 129:484-7.
[14] Buttery JE. Is the Watson-Schwartz screening method for porphobilinogen reliable? Clin Chem 1995;41:1670-1.
[15] Deacon AC, Peters TJ. Identification of acute porpryria: evaluation of a commercial screening test for urinary porphobilinogen. Ann Clin Biochem 1998;35:726-32.
[16] Poh-Fitzpatick MB. A plasma fluorescence marker for variegate porphyria. Arch Dermatol 1980;116:543-7.
[17] Long C, Smyth SJS, Woolf J, Murphy GM. Detection of latent variegate porphyria by fluorescence. Br J Dermatol 1993; 129:9-13.
[18] Bonkowsky HL, Sinclair PR, Scott E, Sinclair JF. Seizure management in acute hepatic porphyria: risks of valporate and clonazepam. Neurology 1980;30:588-92.
[19] Birchfield RI, Cowger ML. Acute intermittent porphyria with seizures. Anticonvulsant medication--induced metabolic changes. Am J Dis Child 1966;112:561-5.
[20] Magnussen CR, Doherty JM, Hess RA, Tschudy DP. Grand mal seizures and acute intermittent porphyria. The problem of differential diagnosis and treatment. Neurology 1975;25:121-5.
[21] Hahn M, Gildemeister OS, Krauss GL, Pepe JA, Lambrecht RW, Donohue S, et al. Effects of new anticonvulsant medications on porphyrin in cultured liver cells: potential implications for patients with acute porphyria. Neurology 1997;49:97-106.
[22] Tatum WO 4th, Zachariah SB. Gabapentin treatment of seizures in acute intermittent porphyria. Neurology 1995;45:1216-7.
[23] Krauss GL, Simmons-O'Brien E, Campbell M. Successful treatment of seizures and porphyria with gabapentin. Neurology 1995;45(part 1):594-5.
[24] Mustajoki P, Nordmann Y. Early administration of heme arginate for acute porphyria attacks. Arch Intern Med 1993; 153:2004-8.
[25] Dover SB, Moore MR, Fitzsimmons EJ, Graham A, McCall KE. Tin protoporphyria prolongs the biochemical remission produced by heme arginate in acute hepatic porphyria. Gastroenterology 1993;105:500-6.
[26] Brodie MJ, Moore MR, Thompson GG, Goldberg A, Low RAL. Pregnancy and the acute porphyria. Br J Obstet Gynaecol 1977;84:726-31.
[27] Todd DJ. Erythropoietic protoporphyria. Br J Dermatol 1994; 131:751-66.
[28] Fritsch C, Bolsen K, Ruzicka T, Goerz G. Congenital erythropoietic porphyria. J Am Acad Dermatol 1997;36:594-610.
[29] Elder GH, Worwood M. Mutations in the hemochromatosis gene, porphyria cutanea tarda, and iron overload. Hepatology 1998;289-90.
[30] Elder GH. Porphyria cutanea tarda: a multifactorial disease. In: Champion RH, Pye RJ eds. Recent advances in dermatology. No 8. Edinbrugh: Churchill Livingstone, 1990:55-70.
[31] Bengtsson NO, Hardell L. Porphyrias, porphyrins and hepatocellular cancer. Br J Cancer 1986;54:115-7.
[32] Elder GH. Porphyria cutanea tarda. Seminars in Liver Disease 1998;18(1):67-75.
[33] Ashton RE, Hawk JLM, Magnus IA. Low-dose oral chloroquine in the treatment of porphyria cutanea tarda. Br J Dermatol 1981; 111:609-13.
[34] Kordac V, Jirsa M, Kotal P. Agents affecting porphyrin formation and secretion: implications for porphyria cutanea tarda. Semin Hematol 1989;26:16-23.
[35] Mathews-Roth MM. Carotenoids in erythropoietic proto-porphyria and other photosensitive diseases. Ann N Y Acad Sci 1993;691:127-38.
[36] Lee JD, Anvert M. Identification of the most common mutation within the porphobilinogen deaminase gene in Swedish patients with AIP. Proc Nat Acad Sci USA 1991;88:10912-5.
Department of Chemical Pathology and Endocrinology, Guy's and St Thomas's Trust, St Thomas's Campus, London SE1 7EH Helen Thadani specialist registrar
Department of Clinical Chemistry, King's College Hospital, London SE5 9RS Allan Deacon consultant clinical scientist
Department of Clinical Biochemistry, Guy's, King's, and St Thomas's Medical Schools, King's College Hospital, London SE5 9RS
Timothy Peters professor
Correspondence to: T Peters, Department of Chemical Pathology, Guy's, King's, and St Thomas's Medical Schools, King's College Hospital, London SE5 9RS timothy.peters@kcl. ac.uk
BMJ 2000;320:1647-51
COPYRIGHT 2000 British Medical Association
COPYRIGHT 2000 Gale Group