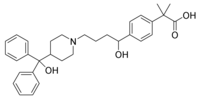
The Food and Drug Administration granted Teva Pharmaceutical Industries tentative approval for its abbreviated new drug application for fexofenadine HC1 tablets in 30 mg, 60 mg and 180 mg dosages. The AB-rated, bioequivalent version of Aventis' antihistamine Allegra is indicated for treating allergic rhinitis and chronic idiopathic urticaria. Teva currently is involved in Paragraph IV litigation with Aventis concerning the product.
In other news, Teva also received tentative FDA approval for its ANDA for tramadol and acetaminophen tablets, 37.5 mg/325 rag, the AB-rated generic equivalent of Ortho McNeil's Ultracet, indicated for the short-term management of acute pain, with total annual branded sales of approximately $338 million. Final approval is expected upon resolution of patent litigation.
Teva also was granted final FDA approval for its ANDA for mirtazapine orally disintegrating tablets in 15 mg, 30 mg and 45 mg dosages. The AB-rated generic equivalent of Organon's Remeron SolTabs is indicated for treating major depressive disorders, with total annual branded sales of approximately $100 million.
Finally, Teva received tentative approval for its ANDA for glimepiride tablets in 1 mg, 2 mg and 4 mg dosages. Final approval is anticipated upon patent expiration Oct. 6. The tablets are the AB-rated generic equivalent of Aventis' antidiabetic agent Amaryl, with annual branded sales of about $340 million.
COPYRIGHT 2005 Reproduced with permission of the copyright holder. Further reproduction or distribution is prohibited without permission.
COPYRIGHT 2005 Gale Group