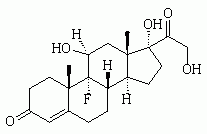
We would like to commend Dr. Jackson (see page 1031) on both his historical review and critical appraisal of the use of etomidate as an anesthetic induction agent. This appraisal is a good summary of the debate that has occurred over the past quarter century in regard to the safety of etomidate as an anesthetic induction agent. The observations of Ledingham and Watt (1) in the early 1980s indicated that etomidate should not be used for long-term sedation in the ICU clue to the mortality cost incurred from long-term adrenal suppression. The effect of etomidate on adrenal function is both dose-dependent and cumulative. A single dose of etomidate will blunt the adrenocortical axis for up to 24 h. The effect of short-term suppression of adrenal synthesis on patient outcome is, however, less clear. This clinical question, although seemingly simple, is quite complex. The net effect of etomidate as an anesthetic induction agent is the sum of several factors. Etomidate has several properties, which makes it, at least in theory, a good first-line anesthetic induction agent. The dose required to achieve unconsciousness is relatively predictable. This hypnotic effect is much more predictable than that with benzodiazepines. The onset of action is fast, essentially in one arm to brain circulation. In addition, etomidate has a short duration of action. Etomidate does not cause histamine release, which is a factor contributing to its relative hemodynamic stability (the reader is referred to an in-depth clinical review of the pharmacology of etomidate (2)). One would expect that these factors would result in a mortality benefit; however, the magnitude of this benefit is unknown. The major concern regarding the use of etomidate is transient adrenal suppression. The unpublished subgroup analysis data presented in the study by Annane et al (3) may provide us with a glimpse of the cost resulting from the adrenal suppression by etomidate. The fact that 68 of the 72 patients (94%) who received etomidate for the induction of anesthesia did not respond to a high-dose cosyntropin stimulation test, is consistent with other published reports of adrenal insufficiency 12 to 24 h after the administration of etomidate. The data from the study by Annane et al (3) seems to indicate a significant mortality cost for etomidate anesthetic induction in septic patients. The mortality rate in the placebo-treated group was 75.7% vs 54.8% for the corticosteroid-treated group. These data indicate that the adrenal insufficiency of sepsis should be treated with the administration of stress doses of corticosteroids. The continued use of etomidate for anesthesia induction would be a clinical conundrum if this mortality effect persisted despite corticosteroid administration. From the available data, we have a presumed, but have not yet measured, mortality benefit incurred from the beneficial effects of etomidate as an anesthetic induction agent. The mortality cost of adrenal suppression by etomidate anesthesia induction seems to be completely offset by corticosteroid administration in those patients who show evidence of adrenal insufficiency. As a result, we feel that the net effect still favors the use of etomidate as an anesthesia induction agent.
The choice of induction agent in hemodynamically labile septic patients is inherently complex, as no perfect anesthesia induction agent exists. Midazolam is typically underdosed when used as the sole anesthetic induction agent, and the time to peak effect is unpredictable. (4) Propofol and barbiturate induction delivers rapid, predictable unconsciousness, with equally predictable hypotension. Ketamine is perhaps the only available agent that provides equally favorable sedation and hemodynamic qualities as those of etomidate, but with its own set of adverse reactions. Dr. Jackson suggests that the induction of unconsciousness and adequate muscular relaxation are measures of the utility of an anesthetic induction agent. We would like to reinforce the notion that these are different issues. Firstly, look at intubation success, and the optimal laryngeal view is best facilitated by muscular relaxation in the form of paralysis. Achieving this level of relaxation with most anesthetic induction agents will only further worsen hypotension during intubation. Furthermore, many of these patients should be considered to have full stomachs, and a rapid-sequence intubation with both an anesthetic induction agent and a paralytic agent should limit the risk of aspiration.
Sepsis continues to be a high-mortality illness. Optimal treatment requires attention to detail and the implementation of many time-dependent therapies starting at the recognition of occult sepsis. We should strive for early empiric antibiotic administration. Early goal-directed resuscitation, as proposed by Rivers et al, (5) has been shown to reduce mortality in this patient population. The treatment of sepsis-induced adrenal insufficiency and tight glycemic control play a significant role in reducing mortality. High-risk patients benefit from the administration of activated protein C. The treatment of sepsis at this time is akin to our current understanding of acute myocardial infarction. There is no magic bullet; rather, several therapies must be quickly brought to bear on this complex pathologic state to maximize the benefit of each intervention while limiting the incurred risk. In our minds, the same holds true for the intubation of a septic patient. This is a high-stakes intervention with a large potential cost if it is not performed well. Significant aspiration or a prolonged period of hypotension may well abolish any benefit from all of the above therapies. We think that etomidate is still a very good agent for the induction of unconsciousness, and when combined with muscle relaxation provides the best scenario for rapid, smooth, hemodynamically stable intubation. The basics of care, the "ABCs," should not be forgotten. Immediate correction of respiratory failure should be performed in a manner that impacts the circulatory system the least. In a Dutch study, Arbous et al (6) demonstrated that about two thirds of the mortality during the induction phase of anesthesia was due to cardiovascular events. This underscores the need for hemodynamic stability during anesthesia induction. Etomidate provides the stability and predictability needed to be a first-line agent. From a practical standpoint, a better anesthetic induction agent is simply not available. As Jackson states it, it is sometimes necessary to stabilize the immediate situation while accepting a future cost. In this ease, the future cost is adrenal suppression. Fortunately, the limited available data indicate that this effect is completely reversed with the administration of corticosteroids. For this reason, we think that all hypotensive septic patients should be treated with stress doses of corticosteroids, particularly if the random (stress) cortisol level is < 25 [micro]g/dL. (7) We recommend initiating therapy with hydrocortisone, 100 mg IV every 8 h, until the results of the stress cortisol level measurements are available. The "cost" of such an approach is likely to be very low, and the potential benefit to be quite high. The cost of such an approach is likely to be very low, and the potential benefit to be quite high.
REFERENCES
(1) Ledingham IM, Watt I. Influence of sedation on mortality in critically ill multiple trauma patients [letter]. Lancet 1983; 1:1270
(2) Bergen JM, Smith DC. A review of etomidate for rapid sequence intubation in the emergency department. J Emerg Med 1997; 15:221-230
(3) Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002; 288:862-871
(4) Sagarin MJ, Barton ED, Walls RM, et al. Underdosing midazolam in emergency endotracheal intubation. Acad Emerg Med 2003; 10:329-338
(5) Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368-1377
(6) Arbous MS, Grobbee DE, van Kleef JW, et al. Mortality associated with anesthesia: a qualitative analysis to identify risk factors. Anaesthesia 2001; 56:1141-1153
(7) Marik PE. Unraveling the mystery of adrenal failure in the critically ill. Crit Care Med 2004; 32:596-597
Holt Murray, MD
Pittsburgh, PA
Paul E. Marik, MD, FCCP
Philadelphia, PA
Dr. Murray is Chief Fellow Department of Critical Care Medicine, University of Pittsburgh Medical Center. Dr. Marik is Chief of Pulmonary and Critical Care Medicine, Thomas Jefferson University.
Correspondence to: Paul E. Marik, MD, FCCP, Chief of Pulmonary and Critical Care Medicine, Thomas Jefferson University, 1015 Chestnut Street, Suite M100 Philadelphia, PA 19107; e-mail: paul.marik@jefferson.edu
COPYRIGHT 2005 American College of Chest Physicians
COPYRIGHT 2005 Gale Group