In this study we examined the effects of exposure to the antiandrogenic fungicide vindozolin (Vz) on the development of two sex-differentiated behaviors that are organized by the perinatal actions of androgens. Pregnant Long-Evans rats were administered a daily oral dose of 0, 1.5, 3, 6, or 12 mg/kg Vz from the 14th day of gestation through postnatal day (PND)3. The social play behavior of juvenile offspring was examined on PND22 and again on PND34 during play sessions with a same-sex littermate. After they reached adulthood, the male offspring were examined with the ex copula penile reflex procedure to assess erectile function. Vz did not produce any gross maternal or neonatal toxicity, nor did it reduce the anogenital distance in male pups. We observed no effects of Vz on play behavior on PND22. However, the 12-mg/kg Vz dose significantly increased play behavior in the male offspring on PND34 compared with controls. The most dramatic increases were seen with the nape contact and pounce behavior components of play. The Vz effect was more pronounced in male than in female offspring. As adults, male offspring showed a significant reduction of erections at all dose levels during the ex copula penile reflex tests. The 12-mg/kg dose was also associated with an increase in seminal emissions. These effects demonstrate that perinatal Vz disrupts the development of androgen-mediated behavioral functions at exposure levels that do not produce obvious structural changes or weight reductions in androgen-sensitive reproductive organs. Key words: antiandrogen, penile reflexes, prenatal exposure, rat, social play, vindozolin. Environ Health Perspect 113:700-707 (2005). doi: 10.1289/ehp.7509 available via http://dx.doi.org/[Online 16 March 2005]
**********
Fungicides are applied to many foods to control plant diseases such as Sclerotinis sclerotiorum (white mold) and Botrytis cinerea (gray mold) (Papadopoulou-Mourkidou 1991). After application, fungicides have been shown to volatilize and circulate through air and water and on untreated foods, increasing their distribution (Baumeister et al. 2002). Consumers cannot readily reduce their exposure because fungicides are not removed from fresh produce by rinsing with tap water (Krol et al. 2000), and commercial processing increases their concentrations (Will and Kruger 1999). Fungicides are also widely used on golf courses, industrial landscapes, lawn turf, and ornamental plants, where they can enter water supplies in contaminated runoff (Haith and Rossi 2003).
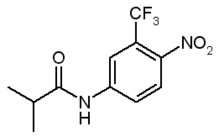
The dicarboximide fungicide vindozolin (Vz) is used in a number of commercial formulations to treat fruits and vegetables such as lettuce, snap beans, canola, and grapes [U.S. Environmental Protection Agency (EPA) 2003]. Vz belongs to a group of environmental endocrine disruptors known as the antiandrogens. These compounds share a common, clearly defined hormone-receptor-mediated mechanism of action. Vz is biotransformed into at least two active metabolites that bind competitively to the human, monkey, and rat androgen receptor (Kelce and Monosson 1995; Kelce et al. 1994). Exposure to antiandrogens during development could have serious effects on sexual development. They are already recognized as one of the factors responsible for the recent increase of hypospadias, a male reproductive disorder where the urethral opening is on the ventral surface of the penis (Baskin et al. 2001; Davis et al. 1998; Egeland et al. 1994; Jensen et al. 1995; Sharpe and Skakkebaek 1993).
To date, most investigations have focused on the impact of the environmental antiandrogens on the development of androgen-sensitive male reproductive organs. For example, adult male rats have reduced anogenital distances (AGDs), reduced seminal vesicle and ventral prostate weights, and lower epididymal sperm counts after perinatal exposure to Vz (Gray et al. 1994; Hellwig et al. 2000). Vz is not the only fungicide that acts as an environmental antiandrogen. Procymidone and iprodione are fungicides that are structurally similar to Vz, and they produce a nearly identical profile of effects on the reproductive system (Gray et al. 1999b; Ostby et al. 1999). All three fungicides have the same final metabolite. However, the doses of Vz that have been shown to affect the weights of reproductive organs in animal studies are quite high, often 5-10 times the U.S.EPA's lowest observed adverse effect level (LOAEL). High doses are associated with measures of gross toxicity such as lowered body weights and increased mortality rate due to granulomas and bladder stones (Gray et al. 1994; Hellwig et al. 2000). Thus, although effects such as reductions in rat reproductive organ weights demonstrate that environmental antiandrogens can produce long-term effects after perinatal exposure, they do not necessarily represent the most sensitive end points. Investigations of the functional effects of low-level environmental antiandrogen exposure are needed to complement the high-dose studies and place organ deficits into the larger context of male reproductive health.
One of the questions examined in the present study was whether much lower levels of Vz during the perinatal period affect reproduction via a disruption of male copulatory behavior. An androgen-sensitive neuromuscular system that is critical for normal male copulatory behavior is the levator ani and bulbocavernosus (BC) skeletal muscles and their motor neuron control centers in the lumbar spinal cord [the spinal nucleus of the BC (SNB)]. In rats, contraction of the levator ani and BC muscles, as well as vascular mechanisms, produces penile erections (Hart and Melese-D'Hospital 1983; Leipheimer and Sachs 1993; Sachs 1982). The sex-specific development of the BC/SNB system is organized during the perinatal period by the non-aromatizable androgen dihydrotestosterone (DHT) (Hart 1979; Thomas et al. 1982). In developing males, the presence of DHT reduces motor neuron death in the SNB and promotes retention of the BC (Mills and Sengelaub 1993). In the adult male, there are two to three times more SNB motor neurons than in females (Sengelaub et al. 1989). However, environmental antiandrogen exposure can disrupt the development of the SNB/BC system. Vz exposure during the perinatal (Wolf et al. 2000, 2004) or peripubertal period (Monosson et al. 1999) significantly reduces the weight of the BC and levator ani muscles in adult males. Other antiandrogens such as procymidone, prochloraz, and linuron also affect the development of the BC muscle (Lambright et al. 2000; Ostby et al. 1999; Vinggaard et al. 2002).
What are the functional implications of an underweight BC muscle that has been affected by Vz exposure? Gray et al. (1994) have shown that adult male rats exposed to perinatal Vz will mount sexually receptive females but are unable to achieve vaginal penetration, suggesting that there is an underlying erectile dysfunction. Other environmental antiandrogens, such as p,p'-dichlorodiphenyldichloroethylene (p,p'-DDE), have already been shown to reduce erectile functions in rats (Brien et al. 2000). Female rats can detect subtle behavioral deficits and prefer to copulate with healthy, dominant males (McClintock et al. 1982). Antiandrogens could therefore affect the reproductive success of a wide range of animal species by altering male copulatory behavior. For instance, female guppies prefer males with high rates of sexual display, and Vz exposure has been shown to significantly reduce male guppy courtship display (Baatrup and Junge 2001; Bayley et al. 2002).
Most functional investigations of environmental endocrine disruptors have focused on the effects of perinatal exposure in adult offspring and have ignored the developmental trajectory of the effects of antiandrogen exposure. Juvenile play is a sexually dimorphic behavior that is an important precursor to adult sexual behavior (Pellis et al. 1992) and dominance relationships (Pellis and Pellis 1992). Males typically engage in more bouts of play and perform more behaviors during bouts than females (Thor and Holloway 1983). Even though they are prominent at different times in the life span, juvenile play and copulation are interconnected. During play, rats perform numerous crawl-over behaviors with same-sex partners. There is a shift of interest in male pups in their preferred play partners during the postnatal day (PND)36-40 period, from male to female (Meaney and Stewart 1981). Older, sexually mature but naive males will perform crawl-overs with females until they achieve a mount with a successful vaginal intromission. After the first intromission, mounting behavior increases and crawl-overs decrease. Adult males that do not have the opportunity to experience normal play during development show excessive play components but little normal copulatory behavior in the presence of sexually receptive females (Gerall et al. 1967; Goldfoot 1977; Gruendel and Arnold 1960; Hard and Larsson 1968). Thus, early environmental factors can affect important neonatal or juvenile social interactions, culminating in aberrant behaviors in adulthood (Dunlap et al. 1978).
The neural mechanisms for play are potential targets for environmental antiandrogens because they are organized in part by androgens during the perinatal period. Administration of the androgen receptor antagonist flutamide during the first 10 postnatal days demasculinizes male rat play behavior (Meaney et al. 1983). Other manipulations such as prenatal protein deprivation (Almeida et al. 1996), perinatal genistein exposure (Flynn et al. 2000), prenatal polychlorinated biphenyl (PCB) exposure (Vreugdenhil et al. 2002), or maternal stress during gestation can also demasculinize play behavior (Ward and Stehm 1991). Recently there has been some interest in the effects of perinatal Vz exposure on social play behavior in juvenile subjects. Hotchkiss et al. (2003) exposed neonatal rat pups to 200 mg/kg Vz on PND2 and PND3. This acute, high-dose exposure had long-term consequences for male rats. Juveniles performed significantly fewer chase and pin behaviors during play sessions with a same-sex partner. Female pups were not exposed to Vz in this study, although it is well known that female play can also be altered by neuroendocrine manipulations (Hines 2003; Nordenstrom et al. 2002; Servin et al. 2003). One study that used a chronic, low-level dietary exposure to Vz found that female rats were more sensitive than males (Flynn et al. 2001).
In the present study, pregnant rats were exposed to low doses of Vz through the last third of gestation and for several days after parturition. Play behavior was examined in juvenile male and female offspring. Erectile function in adult males was assessed using the ex copula penile reflex procedure.
Materials and Methods
Breeding and exposure. Long-Evans hooded rats (Harlan, Indianapolis, IN) were allowed to acclimate to the University of Southern Maine Vivarium quarters for 2 weeks before breeding. All rats were fed standard pellet chow (Teklad Global 18% Protein Rodent Diet; Harlan Teklad, Madison, WI) ad libitum and were maintained on a 12-hr light/12-hr dark cycle in a barrier facility room with an ambient temperature of 68 [+ or -] 2[degrees]F and 40-60% humidity.
Groups of three females were housed with stud males, and vaginal smears were examined each morning for the presence of sperm. We regarded a sperm-positive smear as gestation day (GD)0. Pregnant rat dams were placed individually into polycarbonate shoebox cages and assigned to an exposure condition according to a randomized block design. Each block consisted of five groups: 0, 1.5, 3, 6, or 12 mg Vz/kg maternal body weight. Vz (Crescent Chemical Co. Inc., Islandia, NY) was dissolved in corn oil, and the appropriate volume (~0.5-1.5 mL) was administered via gavage from GD14 through PND3 to coincide with the period of sexual differentiation in the rat (Miller et al. 1988). Vz was not administered on the day of parturition (PND0). We chose the doses in order to examine a range below the U.S. EPA's LOAEL of 11.5 mg/kg/day (U.S. EPA 2000a). The adverse developmental event that is associated with the LOAEL is the retention of nipples and areolas in immature male offspring.
We recorded maternal body weights daily during the gestational period. Cages were inspected each morning and afternoon for the presence of litters. Litter size, sex distribution, pup weights, and AGDs were recorded on PND1 and every 4 days thereafter. Using a randomized procedure, litters were culled to eight offspring on PND4, maintaining equivalent sex distributions when possible. After weaning on PND21, offspring were housed with same-sex littermates in plastic cages with filter bonnets. All animal procedures complied with approved institutional animal care protocols and in accordance with National Institutes of Health guidelines (Institute of Laboratory Animal Resources 1996). Animal care and welfare were supervised by a veterinarian and a Registered Laboratory Animal Technologist certified by the American Association of Laboratory Animal Science.
The exposure and rearing procedure yielded a total of 51 viable litters (Table 1). From this cohort, we assigned 11, 11, 8, and 6 pairs of same-sex littermates from the 0-, 3-, 6-, and 12-mg/kg groups, respectively, to the play procedure. Only those litters with at least 2 male offspring and 2 female offspring were assigned to this procedure. The litter was always considered the unit of analysis, and only 1 male and 1 female pair per litter was assigned to the play procedure. For the penile reflex procedure, 11, 7, 11, 10, and 6 male offspring from the 0-, 1.5-, 3-, 6-, and 12-mg/kg groups were assigned, respectively.
Play behavior. We randomly selected two male and two female pups from each litter. Within each same-sex pair, one animal was designated as the "target" and the other animal served as partner. Before data collection, target and partner animals were marked with a nontoxic marker for identification; they were then separated from their littermates. Twenty-four hours later, the target animal and their same-sex partner were placed together for 10 min in a glass aquarium (12 in. wide x 24 in. long x 12 in. high) with clean cage bedding. We filmed their interactions under dim red light with a Canon XL1s digital video camera (Canon, Inc., Lake Success, NY) interfaced to an iMac computer running iMovie software (Apple Computer, Inc., Cupertino, CA). All testing was conducted during the middle of the dark phase of the light/dark cycle. No other animals were present in the room during filming.
We examined play behavior on PND22 and again on PND34 in the same animals. The assessment ages were chosen in order to examine play immediately after weaning on PND21 and immediately before the decline in same-sex play in male rats, which begins during PND36-40 (Meaney and Stewart 1981).
A pair of trained observers later viewed the films using QuickTime software. (Apple Computer Inc.) Observers tabulated the frequency and distribution of the following five behaviors: nape attack (the snout of the target animal makes contact with the nape area of the partner animal; this behavior occurs frequently and often initiates a bout of play behavior); pounce (the target animal lunges forward with its forepaws extended and makes contact with the partner animal); pin (the target animal is positioned on top of the partner animal with its forepaws placed on the partner; the partner animal lies on its back, fully exposing its ventral surface to the target animal); wrestle (the target and partner animal roll and tumble with each other); and mount (a component of the adult male copulatory pattern where the target animal approaches the partner animal from the rear, clasps its flanks, and mounts).
Penile reflex. In rats, reflexive penile erections and movements can be observed if the penile sheath is retracted with light pressure directed at the base of the penis (Hart and Melese-D'Hospital 1983; Sachs and Garinello 1978). Penile reflexes in the rat consist of erections (tumescence followed by detumescence), cupping (the end of the erect glans penis flares out), and flipping (rapid dorsoflexion of the erect penis). Erections serve to extend the penis beyond the penile sheath, a function that is necessary to achieve vaginal penetration during copulation. Penile flipping serves to stretch the vaginal wall and cupping serves to collect coagulating semen and seal the seminal plug against the cervix.
We conducted all erection tests during the middle of the dark phase. Tests lasted for 20 min after the first response or for 15 min in the absence of responses. During each test, animals were restrained in a supine position with their head and upper torso positioned in a darkened, ventilated tube (8.5 x 5.5 x 20 cm) fastened to a plastic base. The darkened tube is anxiolytic, and rats rapidly habituate to brief periods of restraint. The penile sheath was retracted and held in place (Hart and Melese-D'Hospital 1983; Sachs and Garinello 1978). Typically, clusters of penile erections and dorsoflexions (movements or "flips") begin spontaneously within 5-10 min after sheath retraction.
Trained observers recorded the frequency and time distribution of three gradations of erections: E1, reddening and distension of glans; E2, tumescence of the base and tip of glans; and E3, intense erection accompanied by cupping of the tip of glans (Eaton et al. 1991; Hull et al. 1991; Warner et al. 1991). Penile movements, seminal emissions, latency to the first reflex, and the number of response clusters were also determined. We defined a response cluster as any display of responses separated by [greater than or equal to] 15 sec. Seminal emissions were defined as the expulsion of seminal fluid followed by a coagulating plug.
Statistical methods. For the play procedure, the five behaviors were summed and analyzed as total play behaviors per session with repeated-measures analysis of variance (ANOVA). The individual behaviors were also analyzed separately. The litter always served as the statistical unit of analysis, with the exposure level as a between-litter factor and sex and PND as within-litter factors. In cases where there was a significant main effect or interaction involving the exposure factor, Duncan's probe tests were used to make pairwise comparisons. We considered p < 0.05 statistically significant.
For the penile reflex procedure, we tested each animal on 2 consecutive days, and the data from the two sessions were averaged before analysis. If an animal was inactive during one of the sessions, that session was dropped. If an animal was inactive during both sessions, that animal was dropped from the analysis. Only 2 of 47 animals were inactive during both sessions. The averaged behavior was analyzed with one-way ANOVA according to exposure level.
Because the penile erection data showed a clear dose-response relationship with evidence that even the lowest dose of Vz disrupted the behavior, we further examined the data with Benchmark Dose Modeling Software (BMDS; version 1.3.2; U.S. EPA National Center for Environmental Assessment, Washington, DC). BMDS is a useful alternative to the no observed adverse effect level (NOAEL) approach because it uses the entire dose-response relationship and does not involve extrapolations far below experimental observations. We used the BMDS continuous model to calculate benchmark doses that represented the model-estimated control mean, minus proportional deviations equivalent to a 10% (E[D.sub.10]) or 1% (E[D.sub.01]) decrement in behavior. BMDS also provided a 95% lower bound that can be divided by a standard uncertainty factor to calculate a reference dose or generate a margin of exposure.
Results
Maternal and postpartum data. We found no evidence of gross maternal or neonatal toxicity (Table 1), nor were there any exposure-related changes in maternal body weight, pup body weight, or AGD (males, Table 2; females, Table 3). The percentage of male pups that possessed at least one visible areola on PND 12 (control, 18%; 1.5 mg/kg, 50%; 3 mg/kg, 44%; 6 mg/kg, 45%; 12 mg/kg, 33%) was not significant (p = 0.367).
Play behavior. For this procedure, the primary dependent variable was the total number of play behaviors per session. Although we found no significant main effects of the exposure, sex, or PND factors on total play behaviors, there was a significant exposure x PND interaction [F(1,21) = 7.72, p = 0.01]. We also examined the total number of behaviors separately for PND22 and PND34. There was a significant effect of sex on PND22 [males > females; F(1,21) = 9.91, p < 0.01] and a significant effect of exposure on PND34 [F(1,37) = 16.38, p < 0.001]. Probe tests revealed that the male 12-mg/kg and 6-mg/kg Vz groups produced significantly more play behaviors than the did controls on PND34 (Figure 1A). There were no differences between the female exposure groups (Figure 1B).
[FIGURE 1 OMITTED]
Nape contact and pounce variables made the greatest contribution to the significant exposure-related effects on total play behaviors. For nape contacts, there was a significant exposure x PND interaction [F(1,21) = 5.51, p = 0.03]. We also examined the number of nape contacts separately for PND22 and PND34. As with the total play behavior variable, there was a significant main effect of sex on PND22 [males > females; F(1,21) = 11.13, p < 0.01] and a significant main effect of exposure on PND34 [F(1,37) = 16.09, p < 0.001]. Probe tests indicated that the male 12-mg/kg Vz group produced significantly more nape contacts than did the 0- and 3-mg/kg groups on PND34 (Figure 2). For the pounce variable, there was a significant main effect of exposure [F(1,21) = 6.44, p = 0.02]. Data were averaged across sex and age, and probe tests indicated that the 12-mg/kg group pounced more than did controls (Figure 3). There were no exposure-related differences for pin, wrestle, or mount behaviors.
[FIGURES 2-3 OMITTED]
Penile reflex. We found a significant exposure-related decline in total erections per session [F(4,40) = 4.62, p < 0.01; Figure 4] as each of the Vz groups produced significantly fewer erections than controls. The decline in total erections was due primarily to a dose-related decline of E1 or low-intensity erections [F(4,40) = 10.07, p < 0.01] as well as the number of reflex clusters per session [F(4,40) = 3.23, p = 0.02; Figure 5]. The latency to the first penile reflex and the frequency of E2 and E3 responses were not significantly different. Surprisingly, there was a significant increase in seminal emissions [F(4,40) = 7.37, p < 0.01; Figure 6] as the 12-mg/kg group expelled more often than did any of the other groups. This effect was unanticipated because rats do not usually emit seminal fluid during the ex copula procedure.
[FIGURES 4-6 OMITTED]
Benchmark dose modeling. We performed benchmark dose calculations on the total erections per session and the total play behavior in the male offspring on PND34. These two variables were selected because they are the best overall measures of erectile function and play, and consequently, they generalize more readily to humans. A polynomial model provided the best description of the erection data. The corresponding E[D.sub.10] benchmark for erections was 1.23 mg/kg with a 95% lower bound of 0.84 mg/kg (Figure 7). The E[D.sub.01] benchmark was 0.11 mg/kg with a lower bound of 0.08 mg/kg. The linear model provided the best description of the play data. The E[D.sub.10] associated with total play in the male offspring on PND34 was 1.33 mg/kg with a lower bound of 0.77 mg/kg (Figure 8). The E[D.sub.01] for total play was 0.13 mg/kg with a lower bound of 0.08 mg/kg.
[FIGURES 7-8 OMITTED]
Discussion
The results of this study clearly demonstrate that social and reproductive behaviors in the rat are disrupted by exposure to low doses of Vz during the perinatal period. Maternal doses of 12 mg/kg, administered from GD14 through PND3, were associated with a significant increase in social play behavior in PND34 offspring. We observed no Vz-mediated differences on PND22, indicating that the effect emerged as offspring matured. The increased play on PND34 was more pronounced in males than in females. In adulthood, male offspring produced significantly fewer penile erections, an effect that was even more sensitive than play behavior because a decrease was noted after maternal doses as low as 1.5 mg/kg.
Although other researchers have reported that high doses of Vz (200 mg/kg) administered to rat pups on PND2 and PND3 reduced play behavior (Hotchkiss et al. 2003), this study is the first to describe play behavior effects near the LOAEL of 11.5 mg/kg/day (U.S. EPA 2000a). Although we did not observe nipple and areola retention in immature male offspring, visual inspection of the data suggests that there was a dose-related trend.
The play behavior procedure used in the present study was more sensitive to low-dose effects than those used in previous investigations, possibly because of methodologic differences. In the present study we examined nape contact, pounce, pin, and wrestle, as well as mount behaviors, whereas previous studies examined only pin (Flynn et al. 2001) or pin and chase behaviors (Hotchkiss et al. 2003). Nape contact, a behavior that often initiates a play bout, was greatly affected by perinatal Vz, and this component was not examined in previous studies. Although we hypothesized that perinatal Vz would demasculinize male offspring and lead to a reduction of play behavior, we actually observed a dose-related increase in play. Exposure to other developmental toxicants such as prenatal morphine (Hol et al. 1996; Niesink et al. 1999), mycotoxins (Ferguson et al. 1997), or phytoestrogens (Flynn et al. 2000) has been associated with increased play, and, as mentioned above, social hyperactivity in juvenile rats is linked to aberrant sexual behavior in adults (Gerall et al. 1967).
In the present study we also examined play behavior at two different time points, an approach that detected the apparently transient or age-specific effect of perinatal Vz. In rats, the ontogeny of play is characterized by an inverted U-shaped function that peaks between PND32 and PND40 (Panskeep 1980; Spear and Brake 1983; Ward and Stehm 1991). Male behaviors peak earlier, during PND26-35, with female behaviors peaking during PND36-40 (Meaney and Stewart 1981). The increased play observed in the PND34 exposed males could be interpreted as a developmental delay. Peripubertal exposure to higher doses of Vz has been shown to delay the age of preputial separation, which is a milestone of puberty in the male rat (Monosson et al. 1999). Typically, as male rats age, they show an increasing preference for female versus male partners, a shift that was not observed in the juvenile Vz males. The behavior of the exposed males actually resembled the female offspring, who performed more play on PND34 than on PND22. Future studies should examine additional time points to better characterize the age-dependent nature of the effects of Vz on play. Alternatively, the increased play in the exposed offspring could be due to greater sensitivity to social isolation. Because social deprivation is often viewed as a means of increasing play motivation, this hypothesis could be explored in future studies that compare play after different periods of deprivation. In normal juvenile rats, play solicitation increases after longer periods of deprivation (Thor and Holloway 1983).
Finally, it might be the case that we found significant effects at lower doses in the present study because the offspring were exposed during gestation and the neonatal period via maternal dosing with the gavage procedure. Although many play behavior studies have focused on the role of androgens during the neonatal period, perhaps because of the ease of working with newborn versus fetal rats, the available evidence suggests that the critical period for the differentiation of play begins late in gestation and continues through PND10 (Meaney et al. 1983; Ward and Stehm 1991). Data on the effects of prenatal morphine suggest that the onset of the critical period for play is GD16 (Niesink et al. 1999).
A survey of the developmental toxicology literature indicates that the reduction of erections measured in the present study is one of the most sensitive outcomes observed to date in a perinatal Vz study. Earlier work found that reduced AGD in male neonates and nipple retention occurred after exposure to maternal doses as low as 3.125 mg/kg, whereas at least 50 mg/kg was required to affect ventral prostate weight and increase the incidence of hypospadias (Gray et al. 1999a). Although behavior analysis was not an objective in these earlier investigations, during copulation, exposed males mounted but were unable to achieve vaginal penetration (Gray et al. 1994). In adult male rats, a number of manipulations can produce similar effects, including castration (Leipheimer and Sachs 1993), lesions of the medial preoptic area (Everitt 1990), or microinjection of dopamine antagonist drugs (Pfaus and Phillips 1991). In developing males, prenatal exposure to antiestrogens (Matuszczyk and Larsson 1995) also appears to impair copulatory performance without disturbing sexual motivation. All of these procedures produce structural or functional changes in the erectile system (Hull et al. 1992; Monaghan et al. 1993; Warner et al. 1991).
The Vz-exposed males showed a selective reduction of low-intensity (E1) erections. In this regard, the exposed offspring resemble males castrated as adults, which also show an early reduction of E1 erections (Leipheimer and Sachs 1993). The behavior of the exposed offspring is also reminiscent of male rats that have been administered serotonin receptor agonists (Mas et al. 1985) or agents that block the synthesis of nitric oxide (Hull et al. 1994). Both of these treatments reduce erections and increase seminal emissions. Perhaps the most parsimonious explanation of the differential regulation of penile erections versus seminal emissions has been offered by Hull and others. In a series of drug microinjection studies, this group has demonstrated that pharmacologic stimulation of dopamine D2 receptors in the medial preoptic area decreases the frequency of erections while increasing seminal emissions (Bazzett et al. 1991). Stimulation of D2 receptors in the paraventricular nucleus also facilitates seminal emission (Eaton et al. 1991; Pehek et al. 1989). On the other hand, stimulation of D1 receptors in the medial preoptic area has the opposite effect and occurs at much lower doses (Hull et al. 1992). Because the functional integrity of dopamine systems in this part of the brain is maintained by circulating testosterone (Du and Hull 1999), an environmental antiandrogen such as Vz might disrupt the development of these complex interactions.
As mentioned above, animal studies indicate that fetal males are far more sensitive to environmental antiandrogens than adults. Results from maternal stress studies shed some light on the likely developmental mechanisms affected by environmental antiandrogens. Maternal stress during the last week of pregnancy lowers the surge of plasma testosterone that is normally present in male rat fetuses during GD18 and GD19 (Ward and Weisz 1984). Attenuation of the GD18-19 surge is associated with impaired sexual behavior in adulthood (Dunlap et al. 1978; Ward and Reed 1985). This testosterone surge also exerts an organizational effect on the muscle and spinal cord mechanisms that control penile erections in adulthood (Grisham et al. 1991). Perinatal androgens serve to rescue SNB motor neurons from programmed death (Sengelaub et al. 1989), a process that could be blocked by an antiandrogen like Vz. In the present study, animals were exposed to Vz from GD14 through PND3 in order to compare our results with previous perinatal Vz investigations. However, because differentiation of spinal cord motor neurons continues until PND10 (Mills and Sengelaub 1993) and the weight of the adult BC muscle is the most sensitive to Vz exposure during the GD16-17 period (Wolf et al. 2000), it is likely that the toxic window for Vz on erectile function spans the GD16-PND10 period. Thus, it appears that the critical periods for masculinization of erectile function and play behavior in the male rat are the same. As of yet, no one has examined the effects of environmental antiandrogen exposure during this entire perinatal sensitive period. It may be the case that social play and erectile functions are responsive to even lower doses of Vz, if an exposure were to span the GD 16-PND 10 period.
No previous studies explicitly link Vz to human erectile dysfunction. However, Vz and other antiandrogenic fungicides are used in agriculture, they may be responsible for the recently noted link between pesticide exposure and erectile dysfunction in otherwise healthy men. Specifically, pesticide-exposed men had a significantly higher incidence of complete impotence, showing little to no change from baseline flaccidity on measures of penile rigidity, tumescence, frequency, and duration (Oliva et al. 2002). Occupational exposure to stilbene has also been associated with an increase in self-reported impotence and decreased libido (Quinn et al. 1990; Whelan et al. 1996). Stilbene is a component of textile finishing agents and detergents, and it is structurally similar to the synthetic estrogen diethylstilbestrol. Both of these clinical studies examined the effects of exposure during adulthood. The long-term effects in men after perinatal exposure are unknown.
It is estimated that children 1-6 years of age are exposed to 0.167 mg Vz/kg body weight/day (U.S. EPA 2000b). Given this chronic exposure estimate, a 2-year-old boy who weighs 13 kg [Centers for Disease Control and Prevention (CDC) 2000] would consume an average of 2.17 mg Vz/day, whereas a 6-year-old with a body weight of 21 kg would consume an average of 3.51 mg Vz/day. Both of these estimated daily intakes exceed the E[D.sub.10] benchmark doses associated with altered juvenile play behavior and erectile dysfunction in our animal model. Typically, the U.S. EPA would divide the 95% lower bound by 100 to calculate a reference dose. If this practice were applied to the juvenile play behavior or erectile data, the average daily intake of Vz would exceed the reference doses based on these data by more than two orders of magnitude. It should also be pointed out that humans are exposed to multiple compounds on a chronic basis, whereas this study examined only Vz, which was administered during a limited period of development. The cumulative effects of chronic exposure to multiple compounds and their metabolites are unknown. Lastly, our benchmark doses should be interpreted as conservative estimates because they are based on maternal doses. The actual amount of Vz and/or metabolite that entered our fetal or neonatal subjects is unknown, although the level was certainly lower than the applied maternal dose.
In conclusion, the results of this study demonstrate that the effects of perinatal exposure to an environmental endocrine disruptor can be observed throughout the life span, provided that age-appropriate, sex-specific end points are examined. Low doses of Vz administered during the GD14-PND3 period significantly increased social play behavior in juvenile male rat offspring. These results are interesting in light of recent findings in humans that higher prenatal levels of PCBs have been associated with less masculinized play behavior in Dutch schoolboys and more masculinized play in schoolgirls (Vreugdenhil et al. 2002). Even lower doses of Vz reduced the erectile response in adult male offspring. Because men who work with agricultural chemicals are more likely to experience erectile dysfunction (Oliva et al. 2002), it is quite possible that some of the relevant agrochemicals are antiandrogenic fungicides.
CORRECTION
The AGD data for control females were incorrect in Table 3 of the original manuscript published online, but they have been corrected here.
REFERENCES
Almeida BS, Tonkiss J, Galler JR. 1996, Prenatal protein malnutrition affects the social interactions of juvenile rats. Physiol Behav 60:197-201.
Baatrup E, Junge M. 2001. Antiandrogenic pesticides disrupt sexual characteristics in the adult male guppy (Poecilia reticulata). Environ Health Perspect 109:1063-1070.
Baskin LS, Himes K, Colborn T. 2001. Hypospadias and endocrine disruption: is there a connection? Environ Health Perspect 109:1175-1183.
Baumeister M, Steep M, Dieckmann S, Melzer O, Kloppel H, Jurling H, et al. 2002. Transfer of the fungicide vinclozolin from treated to untreated plants via volatilization. Chemosphere 46:75-82.
Bayley M, Junge M, Baatrup E. 2002. Exposure of juvenile guppies to three antiandrogens causes demasculinization and a reduced sperm count in adult males. Aquat Toxicol 56:227-239.
Bazzett TJ, Eaton RC, Thompson JT, Markowski VP, Lumley LA, Hull EM. 1991. Dose dependent D2 effects on genital reflexes after MPOA injections of quinelorane and apomorphine. Life Sci 48:2309-2315.
Brien SE, Heaton JPW, Racz WJ. 2000. Effects of an environmental anti-androgen on erectile function in an animal penile erection model. J Urol 163:1315-1321.
CDC. 2000. 2 to 20 Years: Boys Stature-for-Age and Weight-for-Age Percentiles. Atlanta, GA:Centers for Disease Control and Prevention. Available: http://www.cdc.gov/nchs/data/ nhanes/growthcharts/set1clinical/cj41c021.pdf [accessed 6 April 2005].
Davis DL, Gottlieb MB, Stampnitzky JR. 1998. Reduced ratio of male to female births in several industrialized countries: a sentinel health indicator? JAMA 279:1018-1023.
Du J, Hull EM. 1999. Effects of testosterone on neuronal nitric oxide synthase and tyrosine hydroxylase. Brain Res 836:90-98.
Dunlap JL, Zadina JE, Gougis G. 1978. Prenatal stress interacts with prepubertal social isolation to reduce male copulatory behavior. Physiol Behav 21:873-875.
Eaton RC, Markowski VP, Lumley LA, Thompson JT, Moses J, Hull EM. 1991. D2 receptors in the paraventricular nucleus regulate genital responses and copulation in male rats. Pharmacol Biochem Behav 39:177-181.
Egeland GM, Sweeney MH, Fingerhut MA, Wille KK, Schnorr TM, Halperin WE. 1994. Total serum testosterone and gonadotropins in workers exposed to dioxin. Am J Epidemiol 139:272-281.
Everitt BJ. 1990. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev 14:217-232.
Ferguson SA, Omer VES, Kwon OS, Holson RR, Houston RJ, Rottinghaus GE Jr, etal. 1997. Prenatal fumonisin (FB1) treatment in rats results in minimal maternal or offspring toxicity. Neurotoxicology 18:561-569.
Flynn KM, Delclos KB, Newbold RR, Ferguson SA. 2001. Behavioral responses of rats exposed to long-term dietary vinclozolin. J Agric Food Chem 49:1658-1665.
Flynn KM, Ferguson SA, Delclos KB, Newbold RR. 2000. Effects of genistein exposure on sexually dimorphic behaviors in rats. Toxicol Sci 55:311-319.
Gerall HD, Ward IL, Gerall AA. 1967. Disruption of the male rat's sexual behaviour induced by social isolation. Anim Behav 15:54-58.
Goldfoot DA. 1977. Rearing conditions which support or inhibit later sexual potential of laboratory-born rhesus monkeys: hypothesis and diagnostic behaviors. Lab Anim Sci 27:548-556.
Gray LE, Ostby JS, Kelce WR. 1994. Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat. Toxicol Appl Pharmacol 129:46-52.
Gray LE, Ostby J, Monosson E, Kelce WR. 1999a. Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health 15:48-64.
Gray LE, Wolf C, Lambright C, Mann P, Price M, Cooper RL, et al. 1999b. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p-DDE, and ketaconazole) and toxic substances (dibutyland dithylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health 15:94--118.
Grisham W, Kerchner M, Ward IL. 1991. Prenatal stress alters sexually dimorphic nuclei in the spinal cord of male rats. Brain Res 551:126-131.
Gruendel A, Arnold WJ. 1960. Effects of early social deprivation on reproductive behavior of male rats. J Camp Physiol Psychol 67:123-128.
Baith DA, Rossi FS. 2063. Risk assessment of pesticide runoff from tuff. J Environ Qual 32:447-455.
Hard E, Larsson K. 1968. Dependence of adult mating behavior in male rats on the presence of littermates in infancy. Brain Behav Evolut 1:465-419.
Hart BL. 1979. Sexual behavior and penile reflexes of neonatally castrated male rats treated in infancy with estrogen and dihydrotestosterone. Harm Behav 13:256-268.
Hart BL, Melese-D'Hospital PY. 1983. Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol Behav 31:807-813.
Hellwig J, van Ravenzwaay B, Mayer M, Gembardt C. 2000. Pre- and postnatal oral toxicity of vinclozolin in Wistar and Long-Evans rats. Regul Toxicol Pharmacol 32:42-50.
Hines M. 2003. Sex steroids and human behavior: prenatal androgen exposure and sex-typical play behavior in children. Ann NY Acad Sci 1007:272-282.
Hal T, Niesink M, Ree JMV, Spruijt BM. 1990. Prenatal exposure to morphine affects juvenile play behavior and adult social behavior in rats. Pharmacol Biochem Behav 55:615-616.
Hotchkiss AK, Ostby JS, Vandenbargh JG, Gray LE Jr. 2003. An environmental antiandrogen, vinclozolin, alters the organization of play behavior. Physiol Behav 79:151-156.
Hull EM, Eaton RC, Markowski VP, Moses J, Lumley LA, Loucks JA. 1992. Opposite influence of medial preoptic D1 and D2 receptors on genital reflexes: implications for copulation. Life Sci 51:1705-1713.
Hull EM, Lumley LA, Matuszewich L, Dominguez J, Moses J, Lorrain DS. 1994. The roles of nitric oxide in sexual function of male rats. Neuropharmacology 33:1499-1504.
Hull EM, Weber MS, Eaton RC, Dua R, Markowski VP, Lumley LA, et al. 1991. Dopamina receptors in the ventral tegmental area affect motor, but not motivational or reflexive, components of copulation in male rats. Brain Res 554:72-76.
Institute of Laboratory Animal Resources. 1995. Guide for the Care and Use of Laboratory Animals. 7th ed. Washington, DC: National Academy Press.
Jensen TK, Toppari J, Keiding N, Skakkebaek NE. 1995. Do environmental estrogens contribute to the decline in male reproductive health? Clin Ghem 41:1896-1901.
Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE Jr 1994. Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol 126:276-285.
Kelce WR, Monosson E, Gray LE Jr. 1995. An environmental antiandrogen. Recent Prog Harm Res 50:449-453.
Krol WJ, Arsenault TL, Pylypiw HM, Mattina MJ. 2000. Reduction of pesticide residues on produce by rinsing. J Agric Food Chem 48:4666-4670.
Lambright C, Ostby J, Bobseine K, Wilson V, Hotchkiss AK, Mann PC, et al. 2000. Cellular and molecular mechanisms of action of linuron: an antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicol Sci 56:389-399.
Leipheimer RE, Sachs BD. 1993. Relative androgen sensitivity of the vascular and striated-muscle systems regulating penile erection in rats. Physiol Behav 54:1085-1090.
Mas M, Zahradnik MA, Mertino V, Davidson JM. 1985. Stimulation of spinal sarotonergic receptors facilitates seminal emission and suppresses penile erectile reflexes. Brain Res 342:128-134.
Matuszczyk JV, Larsson K. 1995. Sexual preference and feminine and masculine sexual behavior of male rats prenatally exposed to antiandrogen or antiestrogen. Harm Behav 29:191-206.
McClintock MK, Anisko JJ, Adler NT. 1982. Group mating among Norway rats II. The social dynamics of copulation: competition, cooperation, and mate choice. Anita Behav 30:410-425.
Meaney MJ, Stewart J. 1981. A descriptive study of social development in the rat (Rattus norvegicus). Anim Behav 29:34-35.
Meaney MJ, Stewart J, Poulin P, McEwen BS. 1983. Sexual differentiation of the social play in rat pups is mediated by the neonatal androgen-receptor system. Neuroendocrinology 37:85-90.
Miller RK, Kellogg CK, Saltzman RA. 1986. Reproductive and perinatal toxicology. In: Handbook of Toxicology (Haley TJ, Bernt WO, eds). Washington, DC:Hemisphere Publishing Corporation, 95-104.
Mills AC, Sengelaub DR. 1993. Sexually dimorphic neuron number in lumbrosacral dorsal root ganglia of the rat: development and steroid regulation. J Neurobiol 24:1543-1553.
Monaghan EP, Arjomand J, Breedlove SM. 1993. Brain lesions affect penile reflexes. Harm Behav 27:122-131.
Monosson E, Kelce WR, Lambright C, Ostby J, Gray LE Jr. 1999. Peripubertal exposure to the antiandrogenic fungicide, vinclozolin, delays puberty, inhibits the development of androgen-dependent tissues, and alters androgen receptor function in the male rat. Toxicol Ind Health 15:69-79.
Niesink RJM, van Buren-van Duinkerken L, van Ree JM. 1999. Social behavior of juvenile rats after in utero exposure morphine: dose-time-effect relationship. Neuropharmacology 38:1207-1223.
Nordenstrom A, Servin A, Bohlin G, Larsson A, Wedell A. 2062. Sex-typed toy play behavior correlates with the degree of prenatal androgen exposure assessed by CYP21 genotype in girls with congenital adrenal hyperplasia. J Clin Endocrinol 87:5119-5124.
Oliva A, Giami A, Multigner L. 2002. Environmental agents and erectile dysfunction: a study in a consulting population. J Androl 23:546-550.
Ostby J, Kelce WR, Lambright C, Wolf CJ, Mann P, Gray LE Jr. 1999. The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in viva and in vitro. Toxicol Ind Health 15:80-93.
Panskeep J. 1980. The ontogeny of play in rats. Day Psychobiol 14:327-332.
Papadopoulou-Mourkidou E. 1991. Postharvest-applied agrochemicals and their residues in fresh fruits and vegetables. J Assoc Off Anal Chem 74:745-765.
Pehek EA, Thompson JT, Hull EM. 1989. The effects of intracranial administration of the dopamine agonist apomorphine on penile reflexes and seminal emission in the rat. Brain Res 500:325-332.
Pellis SM, Field VC, Whishaw IQ. 1992. The role of the cortex in play fighting by rats: developmental and evolutionary implications. Brain Behav Evolut 39:270-284.
Pellis SM, Pellis VC. 1992. Juvenilized play fighting in subordinate male rats. Aggress Behav 18:449-457.
Pfaus JG, Phillips AG. 1991. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci 105:727-743.
Quinn MM, Wegman DH, Greaves IA, Hammond SK, Ellenbecker MJ, Spark RF, et al. 1990. Investigation of reports of sexual dysfunction among male chemical workers manufacturing stilbene derivatives. Am J Ind Med 18:55-68.
Sachs BD. 1982. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil 66:433-443.
Sachs BD, Garinello LD. 1978. Interaction between penile reflexes and copulation in male rats. J Camp Physiol Psychol 92:759-767.
Sengelaub DR, Jordan CL, Kurz EM, Arnold AP. 1989. Hormonal control of neuron number in sexually dimorphic spinal nuclei of the rat: II. Development of the spinal nucleus of the bulbocavernosus in androgen-insensitive (Tfm) rats. J Camp Neural 280:630-636.
Servin A, Nordenstrom A, Larsson A, Bohlin G. 2003. Prenatal androgens and gender-typed behavior: a study of girls with mild and severe forms of congenital adrenal hyperplasia. Dev Psychol 39:440-450.
Sharpe RM, Skakkebaek NE 1993. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 341:1392-1395.
Spear LP, Brake SC. 1983. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol 16:83-109.
Thomas DA, Barfield RJ, Etgen AM. 1982. Influence of androgen on the development of sexual behavior in rats I. Time of administration and masculine copulatory responses, penile reflexes, and androgen receptors in females. Horm Behav 16:443-454.
Thor DH, Holloway WR. 1983. Play-solicitation behavior in juvenile male and female rats. Anim Learn Behav 11:173-178.
U.S. EPA. 2000a. Memorandum from WJ Hazel, Health Effects Division, U.S. EPA, to D Scher and S Lewis, Special Review and Reregistration Division, U.S. EPA. Vinclozolin. Revised Human Health Risk Assessment (Chemical I.D. No. 113201,
DP Barcode D265883), 12 May 2000. Washington, DC:U.S. Environmental Protection Agency. Available: http://www. epa.gov/pesticides/reregistration/vinclozolin/ra_2.pdf [accessed 7 April 2005].
U.S. EPA. 2000b. Vinclozolin. Results of Revised Dietary Risk Assessment. Washington, DC:U.S. Environmental Protection Agency.
U.S. EPA. 2003. Vinclozolin; notice of filing a pesticide petition to establish a tolerance for a certain pesticide chemical in or on food. Fed Reg 68:14628-14635. Available: http://www. epa.gov/fedrgstr/EPA-PEST/2003/March/Day-26/p7246.htm [accessed 7 April 2005].
Vinggaard AM, Nellemann C, Dalgaerd M, Jorgensen EB, Andersen HR. 2002. Antiandrogenic effects in vitro and in vivo of the fungicide prochloraz. Toxicol Sci 69:344-353.
Vreugdenhil HJI, Slijper FME, Mulder PGH, Weisglas-Kuperus N. 2002. Effects of perinatal exposure to PCBs and dioxins on play behavior in Dutch children at school age. Environ Health Perspect 110:A593-A598.
Ward IL, Reed J. 1985. Prenatal stress and prepubertal social rearing conditions interact to determine sexual behavior in male rats. Behav Neurosci 99:301-309.
Ward IL, Stehm KE. 1991. Prenatal stress feminizes juvenile play patterns in male rats. Physiol Behav 50:601-605.
Ward IL, Weisz J. 1984. Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology 114:1635-1644.
Warner RK, Thompson JT, Markowski VP, Loucks JA, Bezzett TJ, Eaton RC, et al. 1991. Microinjection of the dopamine antagonist cis-flupenthixol into the MPOA impairs copulation, penile movements, and sexual motivation in the rat. Brain Res 540:177-182.
Whelan EA, Grejewski B, Wild DK, Schnorr TM, Alderfer R. 1996. Evaluation of reproductive function among men occupationally exposed to a stilbene derivative: II. Perceived libido and potency. Am J Ind Med 29:59-65.
Will F, Kruger E. 1999. Fungicide residues in strawberry processing. J Agric Food Chem 47:858-861.
Wolf CJ, LeBlanc GA, Gray LE Jr. 2004. Interactive effects of vinclozolin and testosterone propionete on pregnancy and sexual differentiation of the male and female SD rat. Toxicol Sci 78:135-143.
Wolf CJ, LeBlanc GA, Ostby JS, Gray LE Jr. 2000. Characterization of the period of sensitivity of fetal male sexual development to vinclozolin. Toxicol Sci 55:152-161.
Nathan K.W. Colbert, Nicole C. Pelletier, Joyce M. Cote, John B. Concannon, Nicole A. Jurdak, Sara B. Minott, and Vincent P. Markowski
Maine Center for Toxicology and Environmental Health, University of Southern Maine, Portland, Maine, USA
Address correspondence to V.P. Markowski, Department of Psychology, University of Southern Maine, 96 Falmouth St., 178 Science Building, Portland, ME 04104-9300 USA. Telephone: (207) 228-8174. Fax: (207) 228-8057. E-mail: markowski@ usm.maine.edu
We thank W.D. Thompson for his assistance with the statistical analyses and S.E. Frankel for his efforts proofreading the manuscript.
This study was supported by a research grant from the Bioscience Research Institute of Southern Maine to V.P.M.
The authors declare they have no competing financial interests.
Received 18 August 2004; accepted 15 March 2005.
COPYRIGHT 2005 National Institute of Environmental Health Sciences
COPYRIGHT 2005 Gale Group