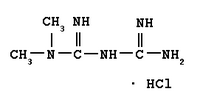
ALPHARETTA, Ga. -- First Horizon Pharmaceutical Corporation (NASDAQ: FHRX), a specialty pharmaceutical company, today announced that it has entered into a definitive agreement to acquire U.S. rights to the type 2 diabetes prescription medication Fortamet(TM) (metformin hydrochloride) and the cholesterol medication Altoprev(R) (lovastatin) from Andrx Corporation ("Andrx"). The Company has also entered into a long-term manufacturing and supply agreement with Andrx.
Fortamet is a patented, once-a-day treatment for type 2 diabetes, and competes in the approximately $6 billion oral diabetes market (based on prescriptions according to IMS Health's National Prescription Audit Plus(TM) data). Fortamet was launched in May 2004 and had reported net sales of approximately $4.5 million in the third quarter of 2004. Altoprev is a patented once-a-day treatment for cholesterol, and competes in the approximately $15 billion statin market (based on prescriptions according to IMS Health's National Prescription Audit Plus(TM) data).
The Company will pay Andrx $50 million at closing and up to $35 million in additional purchase price when Andrx achieves and maintains certain defined manufacturing service levels. Andrx is also entitled to royalties on net sales of both products, as defined. The agreement also contemplates possible future development collaborations. The Company anticipates that it will close the transaction in March or April 2005, subject to approval under the Hart-Scott-Rodino Anti-Trust Improvement Act and the satisfaction of certain other customary closing conditions.
Patrick Fourteau, President and CEO of First Horizon Pharmaceutical commented, "We are excited about the growth potential of these two products, especially Fortamet. Both products participate in well established therapeutic classes and offer specific clinical marketing advantages through their unique dosage forms which fit well with our sales and marketing approach."
Mr. Fourteau further stated, "We intend to initially promote these two products through an expanded sales force of approximately 100 sales representatives which will be deployed in the second quarter of 2005. This increase in our sales force provides us additional resources for the launch of the SkyePharma fenofibrate product, as well as additional flexibility for the promotion of Sular."
In order to finance the transaction, the Company intends to use its available cash and marketable securities. First Horizon was advised on this transaction by UBS Investment Bank and Andrx Corporation was advised by Banc of America Securities. The Company expects that the acquisition will be accretive to earnings.
First Horizon Pharmaceutical Corporation will discuss the acquisition during its fourth quarter financial results conference call on March 2, 2005, beginning at 5 p.m. Eastern Time. Analysts, investors and other interested parties are invited to participate by visiting First Horizon's website, http://www.fhrx.com and entering the Investor Relations page. You may also dial in to the conference call. The dial-in numbers are 1-800-599-9829 for domestic callers and 1-617-847-8703 for international callers. All callers should use the pass code 38451676 to gain access to the conference call. A replay of this conference call will be available by dialing 1-888-286-8010 for domestic callers and 1-617-801-6888 for international callers. All callers should use the pass code 41545554 to gain access to the replay. The replay will be available through March 16, 2005.
First Horizon Background
First Horizon Pharmaceutical Corporation is a specialty pharmaceutical company that markets and sells prescription products with a primary focus on cardiology and women's health. First Horizon has a portfolio that includes 12 branded prescription products of which four are actively promoted to high-prescribing physicians through its nationwide marketing and sales force of approximately 360 representatives. First Horizon's web site address is: http://www.fhrx.com. Please visit First Horizon's website for full prescribing information on First Horizon's products.
Andrx Corporation Background
Andrx Corporation develops and commercializes bioequivalent versions of controlled-release brand name pharmaceuticals, using its proprietary drug delivery technologies, and bioequivalent versions of specialty, niche and immediate-release pharmaceutical products, including oral contraceptives. The Company also develops products for brand pharmaceutical firms related to its controlled-release technology. Andrx also has distribution operations, which purchase primarily generic pharmaceuticals manufactured by third parties and sell them primarily to independent pharmacies, pharmacy chains, pharmacy buying groups and, to a lesser extent, physicians' offices.
Safe Harbor Statement
This press release contains forward-looking statements (rather than historical facts) that are subject to risks and uncertainties that could cause actual results to materially differ from those described. Although we believe that the expectations expressed in these forward-looking statements are reasonable, we cannot promise that our expectations will turn out to be correct. Our actual results could be materially different from and worse than our expectations. With respect to such forward-looking statements, we seek the protections afforded by the Private Securities Litigation Reform Act of 1995. These risks include, without limitation:
Risks related to our proposed acquisition of Fortamet and Altoprev include:
--There is no assurance that the conditions to our acquisition of Fortamet and Altoprev will be satisfied, and we may not acquire Fortamet and Altoprev,
--If we acquire Fortamet and Altoprev, our operating results will be substantially dependent upon the success of Fortamet and Altoprev so that any factor adversely affecting Fortamet and Altoprev could have a material adverse effect on our sales and profits,
--Altoprev is currently experiencing manufacturing issues. If the issues cannot be resolved, this would impact our ability to acquire the product,
--We may incur unexpected costs in integrating Fortamet and Altoprev into our operations,
--Our acquisition of Fortamet and Altoprev will require adjustments to our sales force, which we may not be able to complete successfully or sufficiently rapidly in order to achieve targeted sales of Fortamet and Altoprev,
--The potential growth rate for Fortamet and Altoprev may be limited by slower growth for the class of drugs to which Fortamet and Altoprev belong and unfavorable clinical studies about such class of drugs,
--Strong competition exists in the sale of drugs that treat diabetes and cholesterol, which could adversely affect expected growth of Fortamet and Altoprev sales or increase our costs to sell Fortamet and Altoprev.
Other risks affecting forward-looking statements may also include:
--We may not attain expected net revenues and earnings per share;
--The timing of implementing our plan to increase the number of our sales representatives and the size of such increase may differ from our current expectations;
--The potential growth rate for Sular may be limited by slower growth for the class of drugs to which Sular belongs and the ability of our sales representatives to influence prescribing habits of physicians;
--Sales of our Prenate Elite may not be at the levels that we anticipate;
--We may not be able to protect our competitive position for Prenate Elite from patent infringers;
--Sales of our Tanafed products have been adversely affected by the introduction of competitive products, and an issued FDA notice may cause us to incur increased expenses and adversely affect our ability to continue to market and sell our Tanafed products;
--Introductions by us of line extensions of our existing products may require that we make unexpected changes in our estimates for future product returns and reserves for obsolete inventory which would adversely affect our operating results;
--Our supplier can terminate our rights to commercialize Nitrolingual and the smaller size of this product has not met our expectation;
--A small number of customers account for a large portion of our sales and the loss of one of them, or changes in their purchasing patterns, could result in reduced sales;
--If third-party payors do not adequately reimburse patients for our products, doctors may not prescribe them;
--We rely on operational data obtained from IMS, an industry accepted data source. IMS data may not accurately reflect actual prescriptions (for instance, we believe IMS data does not capture all product prescriptions from some non-retail channels) or trade levels of inventory;
--An adverse judgment in the securities class action litigation in which we and certain directors and executive officers are defendants could have a material adverse effect on our results of operations and liquidity;
--An adverse judgment in our infringement action with Breckenridge Pharmaceutical, Inc. with respect to our Tanafed products, could result in the invalidation of our Tanafed patents, cause us to lose market share for Tanafed products and result in a loss of earnings and profits from Tanafed sales.
--If our products under development fail in clinical studies, if we fail to obtain, or encounter difficulties in obtaining, regulatory approval for new products or new uses of existing products, or if our development agreements are terminated, we will have expended significant resources for no return;
--If the FDA does not approve certain labeling for the cardiovascular product we have licensed from SkyePharma, then our sales of that product may be restricted;
--Our business and products are highly regulated. The regulatory status of some of our products makes these products subject to increased competition and other risks, and we run the risk that we, or third parties on whom we rely, could violate the governing regulations;
--If generic competitors that compete with any of our products are introduced our revenues may be adversely affected; and
--Some unforeseen difficulties may occur.
This list is intended to identify some of the principal factors that could cause actual results to differ materially from those described in the forward-looking statements included herein. These factors are not intended to represent a complete list of all risks and uncertainties inherent in our business, and should be read in conjunction with the more detailed cautionary statements and risk factors included in our other filings with the Securities and Exchange Commission.
Important Safety Information
Fortamet(TM)
Fortamet(TM) is contraindicated in patients with renal disease or renal dysfunction, congestive heart failure requiring chronological treatment, metabolic acidosis, and know allergy to metformin. The most common side effects for Fortamet(TM) include diarrhea, nausea and vomiting.
Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with Fortamet(TM). Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with Lactic acidosis who is taking Fortamet(TM), the drug should be discontinued immediately and general supportive measures promptly instituted.
Altoprev(TM)
Altroprev should not be taken by pregnant or lactating women or those with a known allergy to lovastatin or hypersensitivity to any component of this medication. The most frequent side effects include abdominal pain, muscles aches and diarrhea. All patients should report promptly to their physician any unexplained muscle pain tenderness or weakness. Lovastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected.
COPYRIGHT 2005 Business Wire
COPYRIGHT 2005 Gale Group