Business Editors/Health/Medical Writers
FORT LAUDERDALE, Fla.--(BUSINESS WIRE)--April 28, 2004
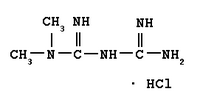
Andrx Corporation (Nasdaq:ADRX) ("Andrx") today announced that the United States Food and Drug Administration ("FDA") has granted final marketing approval of Andrx's New Drug Application (NDA) for Fortamet(TM) (metformin HCl) Extended-Release Tablets, 500 mg and 1000 mg. This NDA provides for the use of Fortamet as an adjunct to diet and exercise in order to lower blood glucose in patients with type 2 diabetes. Andrx is planning to launch Fortamet in May 2004.
Thomas P. Rice, Andrx's Chief Executive Officer, said: "Our 500 mg and 1000 mg strengths of once-a-day Fortamet offer improved convenience and a reduction of pill burden for patients. Our 500 mg tablet is smaller than other currently available metformin tablets, and our 1000 mg tablet is a unique strength that will allow patients to more easily manage their optimal daily dosage requirements. These two strengths of Fortamet are intended to improve patient compliance and further demonstrate the value of Andrx's controlled-release technologies."
Fortamet extended-release tablets employ Andrx's patented single-composition osmotic technology (SCOT(TM)). Fortamet is an extended-release metformin that suppresses hepatic glucose production and improves peripheral insulin sensitivity.
More than 17 million people in the United States have diabetes mellitus, where the most common is type 2 diabetes. Approximately 90% of those diagnosed with diabetes are treated for type 2 diabetes and, over the last decade, the prevalence of type 2 diabetes has increased significantly in all age groups. Type 2 diabetes is a condition characterized by high blood glucose levels caused by either a lack of insulin or the body's inability to use insulin effectively.
The Company also announced today that, in order to avoid possible confusion in the marketplace, it is in discussions with the FDA to market its Altocor product under a new name. These discussions include, but are not limited to, the timing of this name change and the labeling of the product.
About Andrx Corporation
Andrx Corporation is a pharmaceutical company that: develops and commercializes generic versions of controlled-release brand name pharmaceuticals, using the Company's proprietary controlled-release drug delivery technologies, and generic versions of niche and immediate-release pharmaceutical products, including oral contraceptives; distributes pharmaceuticals, primarily generics, manufactured by others as well as manufactured by the Company, primarily to independent pharmacies, pharmacy chains, pharmacy buying groups and physicians' offices; and commercializes brand pharmaceuticals, in some instances using the Company's proprietary controlled-release drug delivery technologies.
Forward-looking statements (statements which are not historical facts) in this release are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. For this purpose, any statements contained herein or which are otherwise made by or on behalf that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the generality of the foregoing, words such as "may," "will," "to," "plan," "expect," "believe," "anticipate," "intend," "could," "would," "estimate," or "continue" or the negative other variations thereof or comparable terminology are intended to identify forward-looking statements. Investors are cautioned that all forward-looking statements involve risk and uncertainties, including but not limited to, Andrx's dependence on a relatively small number of products; licensing revenues; the timing and outcome of patent, antitrust and other litigation; the timing and commercial success of future generic and brand product approvals and launches, including Fortamet; the timing and effects of the change of the Altocor name; whether Andrx will be awarded any market exclusivity period and, if so, the precise dates thereof; government regulation generally; competition; manufacturing capacities, output and quality processes; Andrx's ability to develop and successfully commercialize new products; the loss of revenues from existing products; development and marketing expenses that may not result in commercially successful products; Andrx's inability to obtain, or the high cost of obtaining, licenses for third party technologies; commercial obstacles to the successful introduction of brand products generally, including Fortamet and Cardura XL; the success of Andrx's joint ventures; the impact of returns, allowances and chargebacks; product liability claims; and the absence of certainty regarding the receipt of required regulatory approvals or the timing or terms of such approvals. Andrx Corporation is also subject to other risks detailed herein or detailed from time to time in its filings with the U.S. Securities and Exchange Commission. Andrx disclaims any responsibility to update the statements contained herein.
This release and additional information about Andrx Corporation are also available on the Internet at: http://www.andrx.com.
COPYRIGHT 2004 Business Wire
COPYRIGHT 2004 Gale Group