Business Editors/Health/Medical Writers
FORT LAUDERDALE, Fla.--(BUSINESS WIRE)--Jan. 7, 2004
Andrx Corporation, Fort Lauderdale, FL, ("Andrx"), (Nasdaq:ADRX) and Takeda Chemical Industries, Ltd., Osaka, Japan ("Takeda"), (TSE:4502) jointly announce today that they have entered into an agreement to develop and market a combination product consisting of Takeda's Actos(R) (Pioglitazone) and Andrx's Fortamet(R) (Metformin Extended Release), each of which is administered once-a-day for the treatment of type 2 diabetes. Actos(R) is being marketed in more than 60 countries worldwide while Fortamet(R) was the subject of an October 2003 approvable letter from the United States Food and Drug Administration.
Once approved, this combination product will be manufactured by Andrx, and exclusive marketing rights worldwide will be held by Takeda. Under the agreement, Takeda will be responsible for regulatory approvals for this combination product, and Andrx will receive US$10 million as an initial payment, significant additional milestone payments from Takeda upon the occurrence of certain specified events, as well as a transfer price, a royalty and certain additional performance payments related to Takeda's sale of the combination product.
Richard J. Lane, Andrx's Chief Executive Officer said: "One of Andrx's strengths is formulation technologies. The foundation of this expertise is Andrx's team of talented formulators who have the ability to use this technology to develop products such as this combination of Actos and Fortamet. We are delighted and excited to partner with Takeda, a recognized leader in pharmaceuticals worldwide."
Yasuchika Hasegawa, President & Chief Operating Officer of Takeda said: "We believe this alliance will give Takeda access to Andrx's technology, and will contribute to maximization of the product value and consequently to the further growth of Actos, which is one of our international strategic products and was launched in the US in 1999 and now has yearly sales of more than US$1 billion."
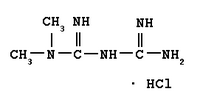
Greater than 17 million people in the United States have diabetes mellitus, where the most common is type 2 diabetes (also known as non-insulin-dependent diabetes mellitus). Approximately 90% of those diagnosed with diabetes are treated for type 2 diabetes, and over the last decade, the prevalence of type 2 diabetes has increased dramatically in all age groups. Type 2 diabetes occurs when the body is unable to effectively use the insulin in the body that is produced. Actos is an oral treatment for type 2 diabetes belonging to the thiazolidinedione class of drugs. It is known as an insulin sensitizer because it directly targets insulin resistance, a condition where the body does not effectively use the insulin it produces. Fortamet is an extended-release metformin that is intended to suppress hepatic glucose production and improve peripheral insulin sensitivity. The combination of Fortamet with Actos is intended to provide a complementary mechanism of action for the treatment of type 2 diabetes, and provide the patient with a more effective option to help achieve adequate blood sugar control.
Fortamet, Andrx's second internally developed brand product, employs Andrx's patented single-composition osmotic technology (SCOT(R)). Andrx has responded to the FDA's approvable letter for Fortamet, and believes that it will receive FDA approval and be able to launch its Fortamet product in the first half of 2004. Andrx anticipates that Fortamet will be specifically indicated for use as a once-a-day therapy to lower blood glucose.
About Andrx
Andrx Corporation develops and commercializes: bioequivalent versions of controlled-release brand name pharmaceuticals, using its proprietary drug delivery technologies; bioequivalent versions of specialty, niche and immediate-release pharmaceutical products, including oral contraceptives; and brand name or proprietary controlled-release formulations of existing immediate-release or controlled-release drugs where it believes the application of Andrx's drug delivery technologies may improve the efficacy or other characteristics of those products. Andrx also has distribution operations, which purchase primarily generic pharmaceuticals manufactured by third parties and sell them primarily to independent pharmacies, pharmacy chains which do not maintain their own central warehousing facilities, pharmacy buying groups and, to a lesser extent, physicians' offices.
About Takeda
Takeda is a research-based global company with its main focus on pharmaceuticals. As the largest pharmaceutical company in Japan and one of the industry's leaders worldwide, Takeda is committed to strive toward better health for individuals and progress in medicine by developing superior pharmaceutical products. Takeda is actively dedicated to enhance its pipeline for future growth through alliance as well as in-house R&D activities.
Forward-looking statements (statements which are not historical facts) in this release are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. For this purpose, any statements contained herein or which are otherwise made by or on behalf of the Company that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the generality of the foregoing, words such as "may," "will," "to," " plan," "expect," "believe," "anticipate," "intend," "could," "would," "estimate," or "continue" or the negative other variations thereof or comparable terminology are intended to identify forward-looking statements. Investors are cautioned that all forward-looking statements involve risk and uncertainties, including but not limited to, whether and when the Pioglitizone/Metformin Extended Release combination product will be filed with the FDA and launched and the commercial success thereof; the timing and outcome of litigation and other future product launches; government regulation generally; competition; manufacturing capacities and output; commercial obstacles to the successful introduction of brand products generally; product liability claims; rising costs and availability of insurance, including product liability insurance; and the absence of certainty regarding the receipt of required regulatory approvals or the timing or terms of such approvals. Andrx Corporation is also subject to other risks detailed herein or detailed from time to time in its filings with the U.S. Securities and Exchange Commission, including, but not limited to, the Company's Annual Report on Form 10-K for the year ended December 31, 2002 and Forms 10-Q for the quarters ended March 31, 2003, June 30, 2003 and September 30, 2003. Andrx disclaims any responsibility to update the statements contained herein.
This release and additional information about Andrx/Takeda are also available on the Internet at: http://www.andrx.com. and http://www.takeda.com, respectively.
COPYRIGHT 2004 Business Wire
COPYRIGHT 2004 Gale Group