Business Editors/Health/Medical Writers
DUBLIN, Ireland--(BUSINESS WIRE)--March 30, 2004
Elan Corporation, plc today announced an agreement with Vernalis plc for the termination of the development and license agreements between Elan and Vernalis regarding Frova(TM) (frovatriptan). Vernalis agreed to purchase Elan's commercialisation rights in North America for Frova.
Kelly Martin, Elan's president and chief executive officer, said, "This transaction allows Elan to further align against our strategic architecture in research, development and marketing. We will focus our resources on preparing for the expected launch of our late-stage pipeline candidates, Antegren for multiple sclerosis and Crohn's disease, and Prialt for pain."
Under the terms of the agreement, Vernalis will pay Elan a total of approximately $55 million for rights to frovatriptan in North America, comprising the following payments. Upon closing, Elan will receive $5 million; on December 31, 2004 and December 31, 2005, Elan will receive payments of $20 million and $25 million respectively; and no later than December 31, 2004, Elan will receive a payment for its Frova inventory, estimated at approximately $5 million. Additionally, Elan's co-promotion agreement with UCB Pharma, Inc. will be terminated at closing, and Elan will pay UCB about $10 million as a result of the termination.
For the full-year 2003, Elan recorded net revenue and gross profit for Frova of $37.5 million and $8.1 million, respectively. The carrying value of the Frova intangible asset is approximately $23 million.
The sale proceeds will be used for development and launch of late-stage pipeline candidates.
The completion of the transaction is subject to the approval of Vernalis' shareholders, U.S. anti-trust clearance if required, third party consents and other customary conditions. The transaction is expected to close before the end of the second quarter of 2004.
About Frova
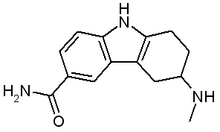
Frova is a prescription medicine used for acute treatment of migraine attacks in adults. It is in the class of drugs called selective serotonin receptor agonists.
About Elan
Elan Corporation, plc is a neuroscience-based biotechnology company that is focused on discovering, developing, manufacturing and marketing advanced therapies in neurology, autoimmune diseases, and severe pain. Elan (NYSE: ELN) shares trade on the New York, London and Dublin Stock Exchanges.
Safe Harbor/Forward Looking Statements
This news release contains forward-looking statements that involve risks and uncertainties and reflects Elan's judgment as of the date of this release. Actual events or results may differ from Elan's expectations. For example, the proposed transaction may not close. In addition, the late stage pipeline candidates mentioned in this news release may never be launched. A further list of risks, uncertainties and other matters can be found in Elan's Annual Report on Form 20-F for the fiscal year ended December 31, 2002, and in its Reports of Foreign Issuer on Form 6-K. Elan assumes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
COPYRIGHT 2004 Business Wire
COPYRIGHT 2004 Gale Group