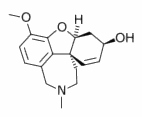
The acetyl cholinesterase inhibitor galantamine improved cognitive, functional, and behavioral symptoms in patients with mild to moderate Alzheimer's disease, reported Dr. P.N. Tariot of the University of Rochester Medical Center and other members of the Galantamine USA-10 Study Group.
Gradual escalation of the dose minimized the side effects, making the drug more tolerable for users, they said (Neurology 54[12]:2269-76, 2000).
Of the 978 patients with probable mild to moderate Alzheimer's disease who participated in this multicenter, parallel-group, placebo-controlled, double-blind randomized trial, 779 (80%) completed the study.
A dose-response benefit on cognitive function was noted at the end of the 5-month treatment period in the patients treated with galantamine (Reminyl), compared with patients on placebo.
A 1.7-point increase on the cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS-cog) was noted in those taking 8 mg/day; a 3.3-point increase was noted among those taking 16 mg/day; and a 3.6-point increase occurred in those on 24 mg/day of galantamine. These improvements were significant for the two higher doses. There was deterioration of 1.8 points in the placebo group.
Also noteworthy was the fact that patients taking 16 mg/day and 24 mg/day maintained their functional ability as assessed by the Activities of Daily Living (ADL) inventory.
Galantamine also had a significant beneficial effect on behavioral symptoms in the two higher dose groups, compared with placebo, Dr. Tariot and associates reported.
The drug was well tolerated. Almost all of the patients were on concomitant medication for other controlled chronic health problems such as hypertension, mild heart failure, diabetes, or hyperthyroidism.
The majority of adverse events, mostly gastrointestinal in nature, were mild. The drug may have been well tolerated because of the gradual way in which the higher doses were introduced. One group of patients was placed on 8 mg/day for 4 weeks before having the dose increased to 16 mg/day for 17 weeks. Another group had the dose begun at 8 mg/day for 4 weeks, followed by 16 mg/day for another 4 weeks, and finally 24 mg/day from week 9 to week 21.
Drug Update: Treating Alzheimer's Disease
Mitchel L. Zoler
Sherry Boschert
The three drugs approved to treat mild or moderate Alzheimer's disease--donepezil, rivastigmine, and tacrine--are all cholinesterase inhibitors. Cholinesterase is an enzyme that breaks down the neurotransmitter acetylcholine, which plays a role in memory and thinking.
Cholinesterase inhibitors are the current mainstays of treatment, along with nonpharmacologic approaches, but these drugs are not a cure, and they do not prevent eventual cognitive decline. They can, however, delay decline or entry into nursing homes among the patient population as a whole. In a small number of patients, they produce mild, transient clinical improvements.
The newest cholinesterase inhibitor, rivastigmine, has not been compared head to head with donepezil, the most common treatment. Donepezil has once-daily dosing; rivastigmine is given twice daily. Both lack the potential liver toxicity and four-times-a-day dosing that have virtually eliminated tacrine's use. If either donepezil or rivastigmine is ineffective in a patient, it is unlikely that the other will work.
The optimum duration of treatment remains unknown. Physicians must weigh the drug's cost against an assessment of whether the patient has stabilized on treatment. Many continue treatment as long as caregivers or family members feel the patient is not getting worse and the drug is tolerated.
In a recent study of mild to moderate Alzheimer's disease, adding high-dose vitamin E to donepezil therapy slowed the progression to dementia, institutionalization, and death but had no impact on cognitive ability. Theoretically vitamin E can interfere with blood dotting, so use it cautiously in patients taking other agents with anticoagulant activity.
Gingko biloba is used by many patients with suspected Alzheimer's disease. A few small studies report that it may have a mild therapeutic effect. A larger study that had some methodologic problems suggested a modest benefit. Most studies used a dosage of 240 mg/day
Experts generally take a neutral stance, neither recommending G. biloba nor telling patients not to take it. A large trial is studying the herb's potential for preventing Alzheimer's disease.
Estrogen replacement therapy and anti-inflammatory agents have been suggested to help prevent Alzheimer's disease, but they do not seem to help patients with Alzheimer's. NSAID treatment does not have enough good data to support its use and may be associated with side effects, but some physicians prescribe it anyway. Treatment with low-dose prednisone does not help Alzheimer's patients, and it may make them worse.
(*.)Cost is based on the average wholesale price for a 100-unit container, or closest available size, of the generic formulation, unless otherwise indicated, in the 2000 Red Book.
(**.)Cost is based on the average wholesale price as supplied by the manufacturer for a 100-unit container.
(+.)The comments reflect the viewpoints and expertise of the following sources:
Dr. John C. Morris, professor of neurology, Washington University, St. Louis.
Dr. Trey Sunderland, chief, geriatric psychiatry branch, National Institute of Mental Health, Bethesda, Md.
COPYRIGHT 2000 International Medical News Group
COPYRIGHT 2001 Gale Group