An investigational treatment for patients who have advanced pancreatic cancer but are not candidates for surgery was granted Treatment IND status by FDA last February.
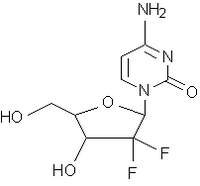
Two clinical trials conducted by the drug's sponsor, Eli Lilly and Co., have suggested that gemcitabine (Gemzar) may effectively treat this cancer. The studies measured tumor shrinkage, survival, and an overall estimate of clinical benefit (pain and ability to function). Both studies showed a small rate of tumor shrinkage (about 7 percent) and about a 25 percent rate of clinical response in the patients. One study showed a small improvement in median survival time (about one and a half months).
The agency's Treatment IND regulations allow drug developers to provide earlier and wider access to investigational therapies for patients with serious diseases for which there is no satisfactory alternative treatment.
About 27,000 people in the United States were diagnosed with pancreatic cancer in 1994. Generally not causing symptoms until late in the disease, it is among the most difficult cancers to treat and is rarely curable. No therapies have been approved specifically for pancreatic cancer.
In clinical tests, the major side effects of gemcitabine have been a decrease in white blood cells (which increases susceptibility to infection), risk of excessive bleeding, and elevation of liver enzymes. Nausea, vomiting, rash, flu-like symptoms, breathing difficulties, and traces of blood and protein in the urine were also reported.
Physicians interested in entering patients in the Treatment IND program may call (1-800) 621-7111 for more information.
COPYRIGHT 1995 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group