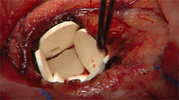
A recently approved implantable wafer is the first technology to deliver an anticancer drug directly to the site of a surgically removed brain tumor in recurrent brain cancer.
Gliadel wafers were approved by FDA on Sept. 24 to treat glioblastoma multiforme, an aggressive type of brain cancer in the malignant glioma class of cancers. Glioblastoma multiforme, which occurs mainly in adults, has been extremely difficult to treat effectively with cancer therapies such as surgery, radiation, and traditional chemotherapy.
Implanted into the cavity of the brain created when a tumor is removed, the wafers--seven or eight of them, depending on the cavity's size--deliver the anticancer drug BiCNU (carmustine) directly to the affected area of the brain. The direct delivery lessens the exposure of the rest of the body to the drug.
In a study of 222 patients with recurrent malignant glioma who had been initially treated with surgery and radiation, the six-month survival rate in those with glioblastoma multiforme who received Gliadel was 56 percent, compared with 36 percent for those who received a placebo. In patients with diagnoses other than glioblastoma multiforme, Gliadel did not affect survival rates. A small 32-patient study supported these results.
Patients should be monitored closely after implantation for possible complications such as seizures, infections, abnormal wound healing, and brain swelling. Approval followed a June 15, 1996, recommendation for approval by FDA's Oncologic Drug Advisory Committee. Since October 1995, Gliadel had been available under a Treatment IND to patients with recurrent malignant glioma.
Gliadel is manufactured by Guilford Pharmaceuticals Inc., of Baltimore.
COPYRIGHT 1996 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group