METHOD OF PREPARATION
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. If tablets are used, pulverize the tablets to a fine powder, or use bulk dolasetron mesylate powder.
4. Slowly add the Ora-Plus and mix, forming a smooth paste and then a uniform suspension or solution.
5. Slowly add the Ora-Sweet SF or strawberry syrup (strawberry fountain syrup, Gordon Food Service, Grand Rapids, Michigan) to volume and mix well.
6. Package and label.
PACKAGING
Package in tight, light-resistant containers.
LABELING
Keep out of reach of children. Use only as directed. Shake well before taking (if tablets are used).
STABILITY
A beyond-use date of 90 days can be used for this preparation.1,2
USE
Dolasetron mesylate oral liquid is used as an antiemetic and antimigraine agent.
QUALITY CONTROL
Quality-control assessment can include weight/volume, pH (pH 4 to 4.5 if the "Ora" family of vehicles is used and 3.6 to 4.6 if strawberry syrup is used), specific gravity, active drug assay, color, clarity, rheological properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth).3
DISCUSSION
Dolasetron mesylate is a selective inhibitor of type 3 serotonergic (5-HT3) receptors and has antiemetic activity. It is used in the treatment of cancer chemotherapy-induced nausea and vomiting and in postoperative nausea and vomiting. It is administered orally or by intravenous infusion.4 Dolasetron is structurally and pharmacologically related to other 5-HT3 receptor antagonists, such as granisetron and ondansetron.
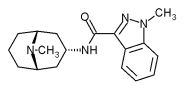
Dolasetron Mesylate USP (C^sub 19^H^sub 20^N^sub 2^O^sub 3^-CH^sub 4^O^sub 3^S, MW 420.48, Anzemet) occurs as a white to off-white powder that is freely soluble in water and propylene glycol, and slightly soluble in alcohol and saline solution.2 Dolasetron (Anzemet) tablets also contain carnauba wax, croscarmellose sodium, hypromellose, lactose, magnesium stearate, polyethylene glycol, Polysorbate 80, pregelatinized starch, synthetic red iron oxide, titanium dioxide, and white wax. The tablets are printed with a black ink that contains lecithin, pharmaceutical glaze, propylene glycol, and synthetic black iron oxide.5
Ora-Plus is an oral suspending vehicle that accepts dilution of up to 50% or more with water, flavoring agents, or syrups and still retains its suspending properties. It has a pH of approximately 4.2 and an osmolality of about 230 mOsm/kg. It is a thixotropic vehicle with Α viscosity of approximately 1,000 cps at 250C. It contains purified water, microcrystalline cellulose, sodium carboxymethylcellulose, xanthan gum, carrageenan, sodium phosphate and citric acid as buffering agents, simethicone as an antifoaming agent, and potassium sorbate and methylparaben as preservatives.6
Ora-Sweet SF sugar-free, alcohol-free syrup is a flavoring vehicle for oral extemporaneous preparations. It has a citrus-berry flavor blend. It is buffered to a pH of approximately 4.2 and may be used alone or in combination with other vehicles. It will tolerate a dilution to 50% with dissolved actives in water or suspending agents and still retain an acceptable taste. It has an osmolality of 2,150 mOsm/kg. It contains water, sodium saccharin, xanthan gum, glycerin, and sorbitol; citric acid and sodium citrate as buffers; methylparaben, propylparaben, and potassium sorbate as preservatives; and flavoring agents.7
Strawberry syrup is prepared using the same method as for cherry syrup. It consists of strawberry juice (475 mL), sucrose (800 g), alcohol (20 mL), and purified water (to make 1000 mL). It should be preserved in tight, light-resistant containers. Exposure to excessive heat should be prevented. It should be labeled to state the Latin binomial name and, following the official name, the part of the plant source from which it was derived. It contains from 1.0% to 2.0% alcohol. It is used as an oral vehicle.2,8
References
1. Johnson CE, Wagner DS, Bussard WE. Stability of dolasetron in two oral liquid vehicles. Am J Health Syst Pharm 2003; 60(21): 2242-2244.
2. US Pharmacopeial Convention, Inc. USP-Pharmacists' Pharmacopeia. Rockville, MD: US Pharmacopeial Convention, Inc.; 2005: 362, 408-431, 683.
3. Allen LV Jr. Standard operating procedure for physical quality assessment of oral and topical liquids. IJPC 1999; 3(2): 146-147.
4. McEvoy GK, ed. AHFS Drug Information-2005. Bethesda, MD: American Society of Health-Systems Pharmacists; 2005: 2802-2804.
5. [No author listed.] Physicians' Desk Reference. 58th ed. Montvale, NJ: Thomson PDR; 2004: 723-726.
6. Ora-Plus [product information]. Minneapolis, MN: Paddock Laboratories, Inc.
7. Ora-Sweet SF [product information]. Minneapolis, MN: Paddock Laboratories, Inc.
8. Ehrenstein E. Aqueous preparations. In: Martin EW, Cook EF, eds. Remington's Practice of Pharmacy. 12th ed. Easton, PA: Mack Publishing Company; 1961:355.
Copyright International Journal of Pharmaceutical Compounding Nov/Dec 2005
Provided by ProQuest Information and Learning Company. All rights Reserved