Haloperidol
Haloperidol (sold as Aloperidin®, Bioperidolo®, Brotopon®, Dozic®, Einalon S®, Eukystol®, Haldol®, Halosten®, Keselan®, Linton®, Peluces®, Serenace®, Serenase®, Sigaperidol®) is a conventional butyrophenone antipsychotic drug. It was developed in 1957 by the Belgian company Janssen Pharmaceutica and submitted to first clinical trials in Belgium in the same year. After being rejected by U.S. company Searle due to side effects, it was later marketed in the U.S. more...
by McNeil Laboratories.
Chemistry
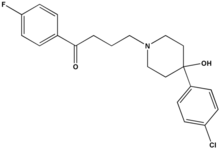
Haloperidol is an odourless white to yellow crystalline powder. Its chemical name is 4--4'-fluorobutyrophenone and its empirical formula is C21H23ClFNO2
Pharmacology
Haloperidol is a neuroleptic, a butyrophenon. Due to its strong central antidopaminergic action, it is classified as a highly potent neuroleptic. It is approximately 50 times more potent than chlorpromazine on a weight basis (50mg chlorpromazine are equivalent to 1mg haloperidol). Haloperidol possesses a strong activity against delusions and hallucinations, most likely due to an effective dopaminergic receptor blockage in the mesocortex and the limbic system of the brain. Too, it blocks the dopaminergic action in the nigrostriatal pathways, which is the probable reason for the high frequency of extrapyramidal-motoric side-effects (dystonias, akathisia, pseudoparkinsonism). It has minor antihistaminic and anticholinergic properties, therefore cardiovascular and anticholinergic side-effects such as hypotension, dry mouth, constipation, etc., are seen quite infrequently, compared to less potent neuroleptics such as chlorpromazine. Haloperidol also has sedative properties and displays a strong action against psychomotor agitation, due to a specific action in the limbic system. It therefore is an effective treatment for mania and states of agitation. Additionally, it can be given as an adjuvant in the therapy of severe chronic pain.
The peripheral antidopaminergic effects of haloperidol account for its strong antiemetic activity. There, it acts at the CTZ (Chemical Trigger Zone). Haloperidol is useful to treat severe forms of nausea/emesis such as those resulting from chemotherapy. The peripheral effects lead also to a relaxation of the gastric sphincter muscle and an increased release of the hormone prolactin, with the possible emergence of breast enlargement and secretion of milk (lactation) in both sexes.
Pharmacokinetics
Oral dosing
Haloperidol is well absorbed after oral dosing. There is a first pass metabolism leading to a reduced oral biovailability of the drug (60 to 70%). Peak plasma-levels are observed after 3 to 6 hours.
I.m. injections
The drug is well and rapidly absorbed and has a high bioavailability. Plasma-levels reach their maximum within 20 minutes after injection.
I.v. injections
The bioavailability is 100% and the very rapid onset of action is seen within about ten minutes. The duration of action is 3 to 6 hours. If haloperidol is given as slow i.v.-infusion, the onset of action is retarded, but the duration prolonged compared to i.v.-injection.
Read more at Wikipedia.org